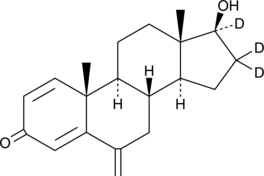
17β-hydroxy Exemestane-d3 |
| Catalog No.GC46471 |
A neuropeptide with diverse biological activities
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: N/A
Sample solution is provided at 25 µL, 10mM.
17β-hydroxy Exemestane-d3 is intended for use as an internal standard for the quantification of 17β-hydroxy exemestane and as a surrogate internal standard for related steroids such as exemestane by GC- or LC-MS. 17β-hydroxy Exemestane is the primary active metabolite of exemestane.1 It is formed by metabolism of exemestane by the cytochrome P450 (CYP) isoforms CYP1A and CYP4A11.2 17β-hydroxy Exemestane is an aromatase inhibitor (IC50 = 69 nM using human placental microsomes) and an androgen receptor (AR) agonist (IC50 = 39.6 nM) that is selective for AR over estrogen receptor α (ERα; IC50 = 21.2 μM).3,4 It stimulates growth of AR- and ERα-positive MCF-7 (EC50 = 2.7 μM) and T47D breast cancer cells (EC50s = 0.43 and 1,500 nM for AR- and ER-mediated growth, respectively) and inhibits proliferation of testosterone-treated aromatase-overexpressing MCF-7aro cells in a concentration-dependent manner.4,5 17β-hydroxy Exemestane (20 mg/kg) inhibits increases in serum cholesterol and LDL levels and prevents decreases in bone mineral density in the lumbar vertebrae and femur, as well as femoral bending strength and compressive strength of the fifth lumbar vertebrae, in ovariectomized rats.1
1.Goss, P.E., Qi, S., Cheung, A.M., et al.Effects of the steroidal aromatase inhibitor exemestane and the nonsteroidal aromatase inhibitor letrozole on bone and lipid metabolism in ovariectomized ratsClin. Cancer Res.10(17)5717-5723(2004) 2.Kamdem, L.K., Flockhart, D.A., and Desta, Z.In vitro cytochrome P450-mediated metabolism of exemestaneDrug Metab. Dispos.39(1)98-105(2011) 3.Buzzetti, F., Di Salle, E., Longo, A., et al.Synthesis and aromatase inhibition by potential metabolites of exemestane (6-methylenandrosta-1,4-diene-3,17-dione)Steroids58(11)527-532(1993) 4.Ariazi, E.A., LeitÃo, A., Oprea, T.I., et al.Exemestane's 17-hydroxylated metabolite exerts biological effects as an androgenMol. Cancer Ther.6(11)2817-2827(2007) 5.Varela, C.L., Amaral, C., Tavares da Silva, E., et al.Exemestane metabolites: Synthesis, stereochemical elucidation, biochemical activity and anti-proliferative effects in a hormone-dependent breast cancer cell lineEur. J. Med. Chem.87336-345(2014)
Average Rating: 5 (Based on Reviews and 13 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















