Bestatin (Synonyms: NK 421, NSC 265489) |
| رقم الكتالوجGC11780 |
An aminopeptidase inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 58970-76-6
Sample solution is provided at 25 µL, 10mM.
Ubenimex(Bestatin) is a specific inhibitor of aminopeptidase B and leucine aminopeptidase. It did not show any inhibition of aminopeptidase A, trypsin, chymotrypsin, elastase, papain, pepsin or thermolysin. Bestatin at 100 pg/ml showed no antibacterial and no antifungal activities. It has low toxicity with no death after intraperitoneal injection of 300 mg/kg to mice1.
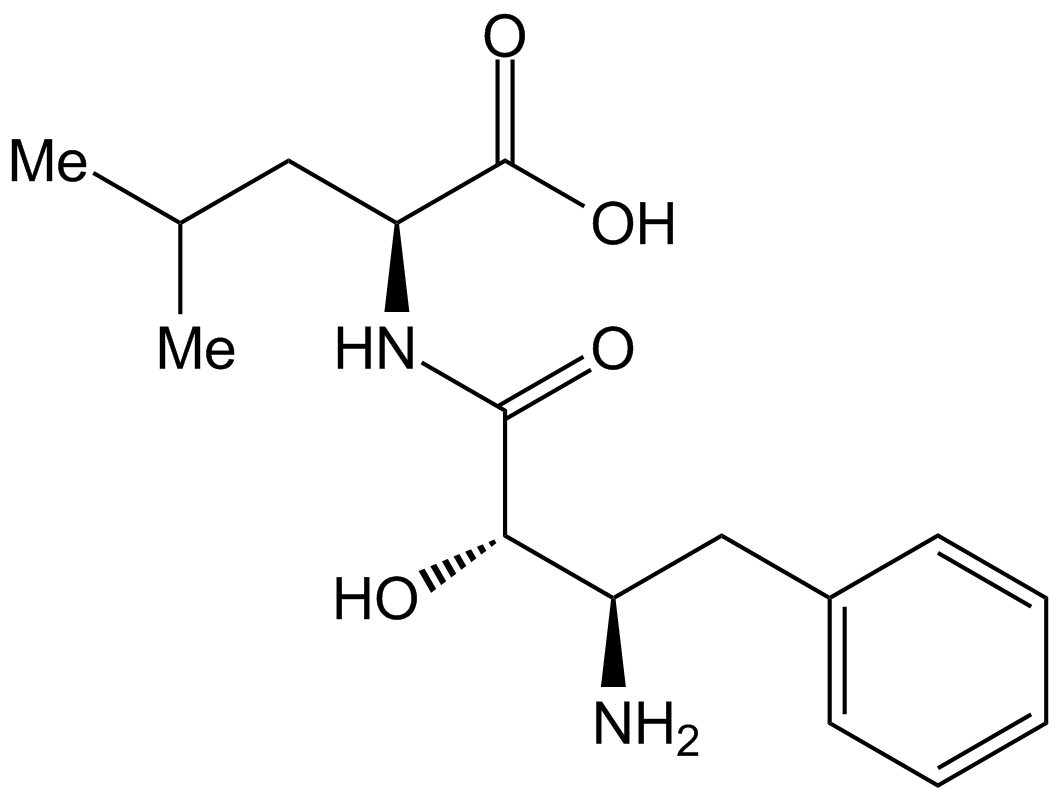
Bestatin isolated from the culture filtrate of Streptomyces olivoreticuli MD976-C72. The structure of bestatin was elucidated to be (2S, 3R)-3-amino-2-hydroxy-4- phenylbutanoyll-(S)-leucine3, 4. Bestatin itself was not hydrolyzed by either of the enzymes, when bestatin was incubated as substrate, L-leucine was not detected by thin-layer chromatography.
Unlike the case of orthophenanthroline, the inhibitory activity of bestatin on aminopeptidase B was not reversed by addition of zinc ion. Bestatin has a pair of adjacent amino and hydroxyl groups, which shows metal-complexing activity5-7. If the inhibitory activity of bestatin is attributable to five-membered chelate ring formation by a pair of adjacent amino and hydroxyl groups of bestatin and a metal ion of the enzyme, the isomers having erythro AHPA, which is difficult to form a chelate ring, are expected not to show inhibitory activity. However, the isomers having erythro-AHPA or (2S, 3S)-AHPA showed marked inhibitory activity. Bestatin and its active isomers are effective due to a mechanism other than a chelating action at the active center8.
References:
1. Umezawa H, Aoyagi T, Suda H, Hamada M, Takeuchi T, Bestatin, an inhibitor of aminopeptidase B, produced by actinomycetes, J Antibiot (Tokyo). 1976 Jan; 29(1):97-9.
2. Umezawa, H., Aoyagi, T., Suda, H., Hamada, M., And Takeuchi, T. 197615. Antibiotics 29, 97.
3. Suda, H., Takita, T., Aoyagi, T., And Umezawa, H. (1976) J. Antibiotics 29, 100.
4. Nakamura, H., Suda, H., Takita, T., Aoyagi, T., Umezawa, H., And Iitaka, Y. (1976) J. Antibiotics 29, 102.
5. Umezawa, S., Tsuchiya, T., And Tatsuta, K. (1966) Bull. Chem. Sot. Japan 39, 1235.
6. Barlow, C. B., And Gijthrie, R. D. (1967) J. Chem. Sot. (C) 1194.
7. Bukhari, S. T. K., Guthrie, R. D., Scott, A. I., And Wrixon, A. D. (1970) Tetrahedron 26, 3653.
8. Suda et al. Inhibition of aminopeptidase B and leucine aminopeptidase by bestatin and its stereoisomer, Archives of Biochemistry and Biophysics, 77, 196-200 (1976)
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















