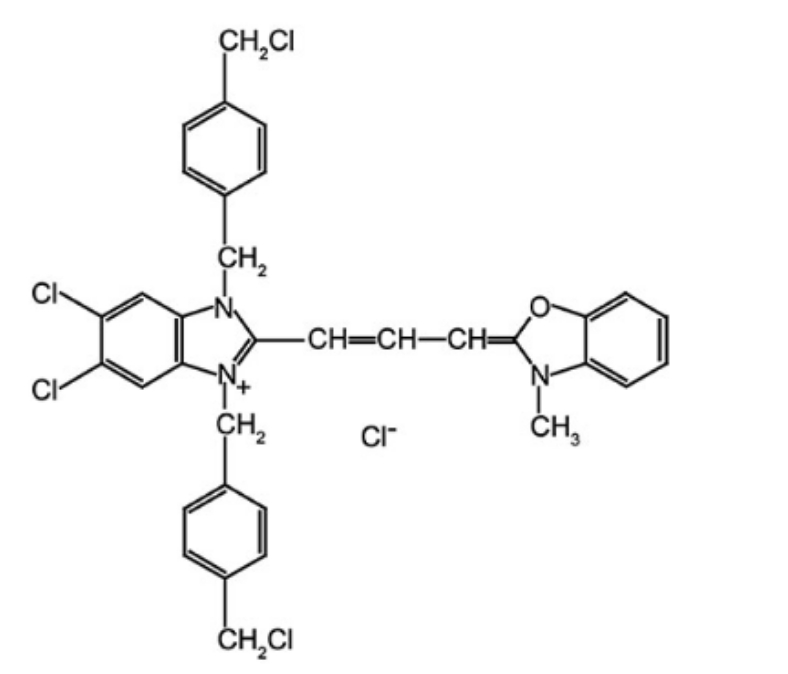
L-3-n-Butylphthalide
Drugs including calcium channel blockers, excitatory amino acid receptor antagonists and free radical scavengers are used for the prevention and treatment of ischemic CVDs such as strokes. Such drugs have side effects as well. There is a urgent need for such class of neuroprotective drugs that has better therapeutic effects and minimum side effects. Such class was discovered under the heading name of Levo 3-n-Butylphthalide (NBP). Such drug has been isolated from celery leaves. Such drugs gain attention because of their ability to increase cerebral blood flow in the ischemic zone. 3-n-Butylphthalide (NBP), a synthetic compound has it’s base on l-3-n-Butylphthalide, extracted from the seeds of Apium graveolens. The approval for 3-n-butylphthalide (dl-NBP) is done by Food and Drug Administration of China in 2002. The major findings proved that 3-n- Butylphthalide is not only beneficial for ischemic stroke but also for long term memory recovery.
Chemical formula: C12H14O2
Molecular weight: 190.2406
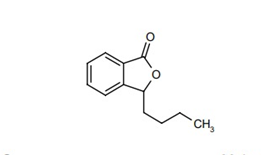
Figure 1: Structural formula of L-3-n-Butylphthalide.
A study was conducted to prove that Brain edema, increased flow of Cerebral Blood and improved neurobehavioral Outcomes Transient Middle Cerebral Artery Occlusion Rats is Attenuated by NBP. Before surgery, 10 min after occlusion, and immediately after reperfusion measurements were taken for blood parameters like including pH, PCO2, PO2, sodium, potassium, and glucose concentration. The results show that no major changes occur in physical parameters before and after tMCAO operation as shown in table 1.
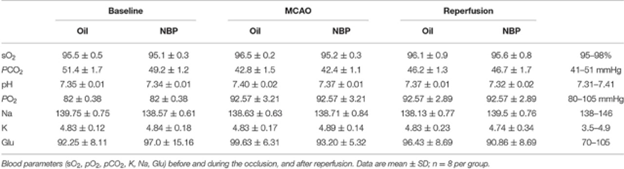
Table 1. Analysis of blood parameters.
Brain edema is caused by blood brain barrier. Measurements for brain edema and brain infarct volume in rats treated with L-3-n-Butylphthalide. were taken at day 1 and 3 after tMCAO. The ischemic infarct area decreased in rats treated with L-3-n-Butylphthalide as compared to that in control as shown by results fig 2A. It was shown that the infarct area in the rat treated with L-3-n-Butylphthalide was limited to striatum while in control it was larger in both cortex and striatum. The results analyzed after days 1 and 3 of tMCAO (p < 0.05) show that L-3-n-Butylphthalide treatment also minimize the volume of brain edema (Figures 2B,C). While, at days 1 and 3 tMCAO (p < 0.01) the neurobehavioral deficiency after ischemic brain injury is also minimize as shown by evaluation of mNSS , (Figure 2D).
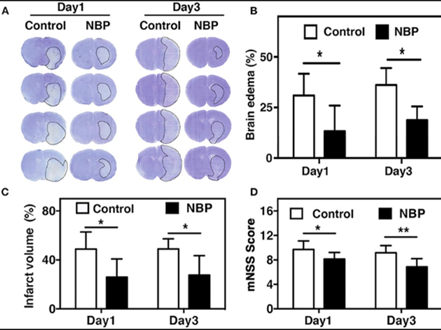
Figure 2. L-3-n-Butylphthalide reduced edema and improved neurobehavioral outcomes in tMCAO rats. (A) at day 1 and 3 after tMCAO the cresyl violet staining in rats reated with L-3-n-Butylphthalide and in control rats is represented by photomicrography. The border of the infarct area is showed by dashed line. At day 1 and 3 after tMCAO semi quantification of brain edema volume is shown in fig 2B and the infarct volume (C). Fig 2D shows the mNSS assessment at day 1 and 3 after tMCAO in the rat treated with NBP and in control rat. Data are mean ± SD; n = 8 per group; *p < 0.05 and **p < 0.01; NBP-treated rats vs. control rats.
BV2 cells are protected from rotenone-induced cell viability injury, mitochondrial dysfunction, and mtDNA leakage by L-3-n-Butylphthalide. The effects of L-3-n-Butylphthalide on rotenone-induced cell viability and mitochondrial damage in BV2 cells are investigated. The CCK-8 assay are used first to measure cell viability. The BV2 cell viability is reduced by 50% by rotenone as compared to normal control group as shown in fig 3b. While the cell viability is increased in rotenone + L-3-n-Butylphthalide group as shown in fig 3b. Consequently, to determine the localization of mitochondria and the mtROS content MitoTracker and MitoSOX fluorescence staining is employed. In rotenone-treated BV2 cells mtROS levels increase while mtROS production is decreased by L-3-n-Butylphthalide (Figure 3(a)). Moreover, the amount of mtDNA leakage is measured by qRT-PCR. The results showed that rotenone caused mtDNA leakage into the cytoplasm, whereas L-3-n-Butylphthalide alleviated this leakage (Figure 3(c)).
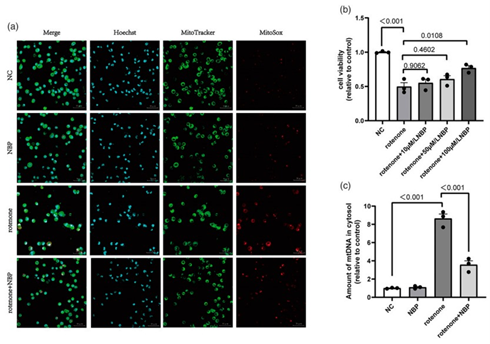
Figure 3: Rotenone-induced cell viability injury, mitochondrial ROS levels and mtDNA leakage is inhibited by L-3-n-Butylphthalide. In BV2 cells mitrocondrial ROS level is detected by (a) MitoSOX (red)/ MitoTracker (green)/Hoechst (blue) staining. (b) In BV cells cell viability was measured by using a CCK8 assay. (c) In BV2 cells the levels of cytoplasmic free mtDNA were detected by using qRT-PCR. Data are expressed as the means ± SEM (n = 3). p value is provided in all histograms.
In vitro, the cyclic GMP-AMP synthase-stimulator of interferon genes pathway activation and inflammation induced by rotenone is inhibited by N-Butylphthalide. To explore the relationship between NBP and the cGAS-STING pathway Western Blot and qRT-PCR. Western Blot is used to detect the levels of cGAS, STING, NfκB, p-NfκB, IκBα, and p-IκBα proteins. Levels of cGAS and STING proteins as well as the ratios of p-NfκB/NfκB and p-IκBα/IκBα is detected by Western Blot while these changes are minimized by NBP (Figures 4(a)–(e)). In BV2 cells, the mRNA level of IL-1β, TNF-α, and IL-6 is increased by rotenone shown by qRT-PCR. Compared with the rotenone group, the mRNA levels of these inflammatory factors were reduced in the rotenone + NBP group (Figures 4(f)–(j)). These findings suggest a new mechanism by which NBP inhibits neuroinflammation.
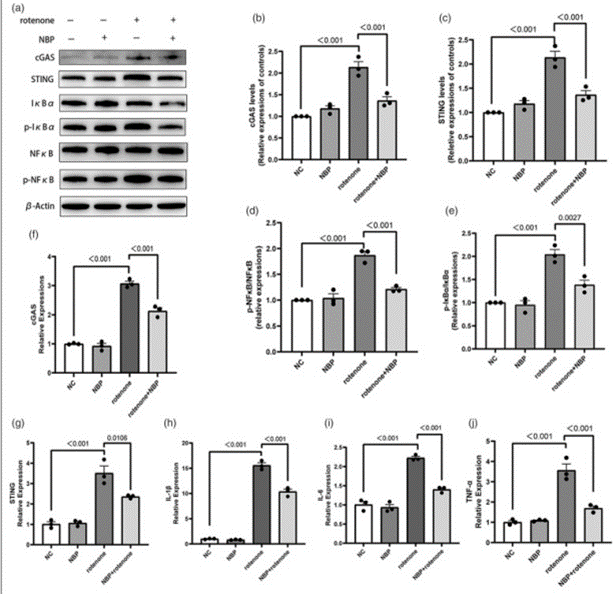
Figure 4: L-3-n-Butylphthalide inhibits the activation of the cGAS-STING signaling and inflammation induced by rotenone in BV2 cells.
For the effective of CVDs L-3-n-Butylphthalide is very effective. In case of ischemic stroke, NBP can reduce neuroinflammation by inhibiting the inflammasome in microglia. In a repeated cerebral ischemia-reperfusion model, NBP showed anti-inflammatory, antioxidant, and neuroprotective effect. Recently, the possible therapeutic effects of NBP in neurodegenerative diseases have garnered increasing attention. The results showed that the inhibition of cGASSTING pathway and alleviating neuroinflammation causes the protective effects in rotenone-induced PD models by NBP. In mice exposed to rotenone, L-3-n-Butylphthalide minimizes the behavioral impairments. While NBP enhances behavioral impairments in mice shows resemblance with PD when exposed to rotenone. In mice treated with rotenone the analysis of rotarod and balance beam tests shows that mice shows impaired motor function evidenced by a reduction in the time spent on the rotarod and increase in the time taken to complete the full-length balance beam (Figures 5(b) and (d)). While the mice treated with both rotenone and NBP the mice shows impaired motor function as shown in fig 5(b) and (d). Based on the results of the T-maze test, the rotenone-treated mice showed impaired spatial memory with reduced accuracy in choosing the correct arm (Figure 5(c)). Compared with rotenone-treated mice, mice subjected to both L-3-n-Butylphthalide and rotenone showed increased accuracy, suggesting an improvement in spatial memory (Figure 5(c)).
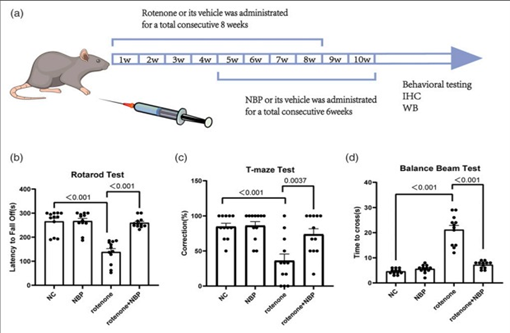
Figure 5: L-3-n-Butylphthalide alleviates the behavioral impairments in rotenone-induced PD mice.
NBP has been shown as a promising candidate for disease modification and the delay of PD progression. This drug and it’s derivatives derivatives been essentially shown to possess antiplatelet aggregation, antithrombotic, antiapoptotic, and antioxidant properties coupled with promotion of neurogenesis and protection of mitochondrial functions. L-3-n-Butylphthalide has the potential to be a precursor to a whole new group of drugs and therapeutic approach to several neurological conditions. This opens a new perspective for future treatment of a wide range of conditions.















Comments