Pembrolizumab
Pembrolizumab, marketed under the brand name Keytruda, is a monoclonal antibody used in cancer immunotherapy. It targets the programmed cell death protein 1 (PD-1) receptor on T cells, a critical checkpoint in the immune system. By inhibiting PD-1, pembrolizumab enhances the immune system's ability to detect and destroy cancer cells. This mechanism of action places pembrolizumab within the class of drugs known as immune checkpoint inhibitors, which have significantly advanced the treatment of various cancers. Molecular formula: C₆₅₃₄H₁₀₀₀₄N₁₇₁₆O₂₀₃₆S₄₆ .Weight: 146648.64 gmol−¹.
The next strategy should be that under severe chronic conditions of stimulation the impact of PD-1 blockade in antigen-specific will be characterized. Herein, for the time period of specifically seven days EBV peptide pool were stimulated with PBMCs, and IFNy and IL-10 will be measured in the supernatant of the cell culture likewise. As per expectation, the level of IFNY and IL-10 will be in less and less quantity as compared to that which is measured while using allogeneic DCs as a stimulus (Fig. 2A-C). As per observation, under the usage of such stimulation conditions, the level of IFNY increase in the PBMC cultures as likewise of IL-10 in the PBMC cultures, which the significant appears when compared with isotype control statistically (Fig. 2C). While comparing individual donor responses, the release of IFNY is increased. This impacts that pembrolizumab was capable of enhancing T cell responses.
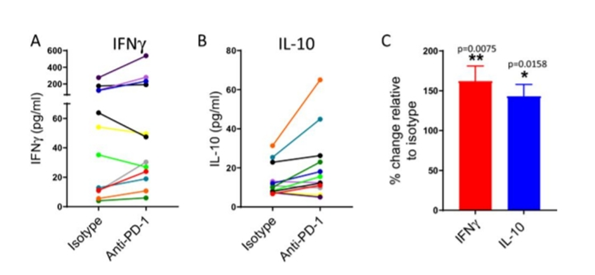
Figure 1: Effects of pembrolizumab on IFNY and IL-10 levels from PBMC cultures stimulated with EBV peptides. Pembrolizumab increases IFNγ and IL-10 levels in cultures of PBMCs stimulated with EBV peptides. Ability of pembrolizumab (anti-PD-1; 1.0 μg/ml) to modify IFNγ (A) or IL-10 (B) levels in cultures of PBMCs stimulated with EBV peptides. (A,B) indicate responses of individual donors (each colour represents an individual donor). © indicates percentage change in response in the presence of pembrolizumab relative to isotype control (mean + SEM, n = 12).
Pembrolizumab therapies, the main action site of which is the interactionary relationship between Programmed cell death protein 1 (PD-1) and its ligands. PD-1, one of the member of CD28 family, shows it’s expression on the surface of activated immune cells e.g T-cells and B-cells. PD-1 shows a high affection for the PD-1, let it to has a competition with internal PD-1 (Figure 3). This enlarges the activity of T cells resulting in it’s immunological response against cancer cells. X-ray crystallographic studies of human and mice revealed the fact that the interaction of PD-1 and PD-L1 is facilitated by the CC' loop and the FG loop of PD-1.
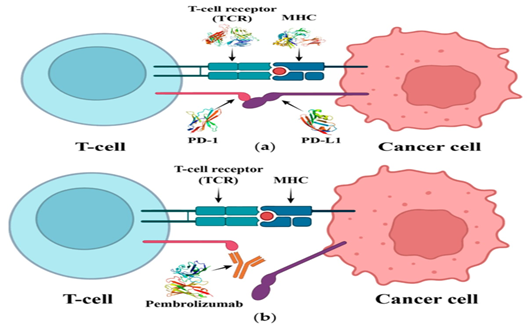
Figure 2: (a) Shows the interacting activities of T cell receptor with MHC. It leads to the activation of the T cell receptor while the interaction profile of PD-1 with PD-L1 cause the inhibition of T cell. (b) Pembrolizumab has an inhibitory effects on the interaction of PD-1 and PD-L1, leading to inhibiting the T cell inhibition.
The KEYNOTE-045 of pembrolizumab compare the pembrolizumab along with the chemotherapy. In the month of June 2018, pembrolizumab was used to treat patients that was PD-L1 positive for cervical cancer. The results of the KEYNOTE-224 trial, approved pembrolizumab as a second line of defense for HCC. The trial for phase ll is done. This included 104 patients of HCC that was pretreated with sorafenib and the response rate is only 17%. 25% of these patients was found to be positive for hepatitis B and C virus. KEYNOTE-010 is a kind of that was proposed to investigate the of benefits of pembrolizumab in the view of other medications. This study proposed the investigatory effect for the potential of pembrolizumab in the persons that was pretreated with platinum based chemotherapy. A round of 35 cycles was done included 1034 patients that were the dose of pembrolizumab either in the quantity of 2 mg/kg given to 345 patients or 10 mg/kg given to 346 patients after every 3 weeks or the dose of docetaxel either in the dose of 75 mg/m2 given to 343 persons after every 3 weeks. When the study was done it is found that a total of 521 patients including 50% of those that were given the quantity of 2 mg/kg of pembrolizumab, 45% of those that were given the quantity of 10 mg/kg of pembrolizumab and 56% of those that were given the docetaxel (Table 1), while the survival rate of the patients was ≥ 50% (Table 2).
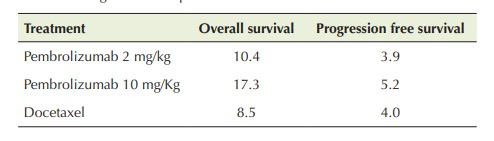
Table 1: Overall survival rate (months) and progression-free survival at different dosages and therapies.
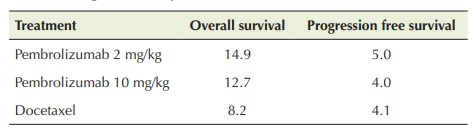
Table 2: Overall survival rate (months) and progression-free survival at different dosages and therapies with TPS PD-L1 ≥ 50%.
Where there is the tremendous effects of pembrolizumab, it has adverse effects as well. The most noted on side effects are fatigue, musculoskeletal pain, rash, diarrhea, pyrexia, cough, decreased appetite, pruritus, dyspnea, constipation, pain, abdominal pain, nausea, and hypothyroidism. Some side effects are not due to single use of pembrolizumab, but in it’s combination with chemotherapy like fatigue/asthenia, nausea, constipation, diarrhea, decreased appetite, rash, vomiting, cough, dyspnea, pyrexia, alopecia, peripheral neuropathy, mucosal inflammation, stomatitis, headache, weight loss, abdominal pain, arthralgia, myalgia, and insomnia. While there are side effects also there are not due to pembrolizumab and it’s combination with chemotherapy but due to the effect of these three (pembrolizumab, chemotherapy and bevacizumab) including peripheral neuropathy, alopecia, anemia, fatigue/asthenia, nausea, neutropenia, diarrhea, hypertension, thrombocytopenia, constipation, arthralgia, vomiting, urinary tract infection, rash, leukopenia, hypothyroidism, and decreased appetite.
Pembrolizumab, an immunotherapy use widely to treat various malignant conditions caused the improvement in the patient’s conditions and increase it’s survival rate. Healthcare workers must take into account the adverse effects as well as the potential benefits of pembrolizumab to decide the treatment profile of the patients with muscular weakness, blepharoptosis, and diplopia. Pembrolizumab represents a significant advancement in the field of cancer immunotherapy. It has ability to govern the body’s immune system to fight cancer and it offers new hope to patients with various types of cancer. As research continues, the potential for pembrolizumab to improve outcomes and extend survival of the patients remains promising and challenging for the scientists .













Comments