Semaglutide
Semaglutide is a polypeptide composed of 31 amino acids related by peptide bonds, with a molecular heaviness of 4114 g/mol. It actions as an agonist for glucagon-like peptide-1 receptors (GLP-1 R) and is functioned in the action of type 2 diabetes. Semaglutide purposes as a hypoglycemic agent, a GLP-1 receptor agonist, an anti-obesity mediator, a neuroprotective mediator, and want for food suppressant. It is confidential as both a polypeptide and a lipopeptide Recent advancements have highlighted the potential of GLP-1 receptor agonists, like semaglutide, in countering the effects of hyperglycemia and situation of ferroptosis due to its antioxidative and anti-inflammatory properties . Despite its well documented benefits in managing diabetes the specific impact of semaglutide on diabetic testicular damage and its ability to inhibit ferroptosis is not fully understood.
Figure 1: Chemical Structure Depiction
Diabetes is linked to male infertility, but its instruments and managements are unclear. This study discovers the effects of Semaglutide on testicular purpose in diabetic mice. Medical records show diabetes affects blood glucose, lipid levels, and sperm quality. Testicular torsion is a serious emergency and a main reason of male infertility, affecting 10%–15% of scrotal circumstances in children. Kisspeptin (KISS1), a hormone from the hypothalamus and testis, has an unclear role in testicular purpose. Semaglutide, a novel GLP-1 analog used for diabetes, was examined for its potential to advance testicular dysfunction caused by ischemia/reperfusion in rats. This education explores how Semaglutide influences the testicular GLP-1/PGC-1α–PPAR-α–KISS1 signaling pathway .
Semaglutide improves glucose metabolism, lipid metabolism and Sperm quality in db/db mice : For the investigation the role of semaglutide on the regulation of metabolic disorders and testicular damage, researchers administrated db/db mice with semaglutide for 8 weeks . Semaglutide and Fer-1 treatment had a significant decrease on the body weight, daily food intake, daily water intake and epididymal fat weight of the db/db mice. Semaglutide treatment also significantly reduced blood glucose of DM mouse model serum, but no difference was seen in the Fer-1 group . Furthermore, the model mice showed a significant decrease in testicular weight, testicular index, testosterone in testis, sperm counts per cauda epididymis and percentage of motile sperm, while semaglutide and Fer-1 treated mice significantly improved testicular function.
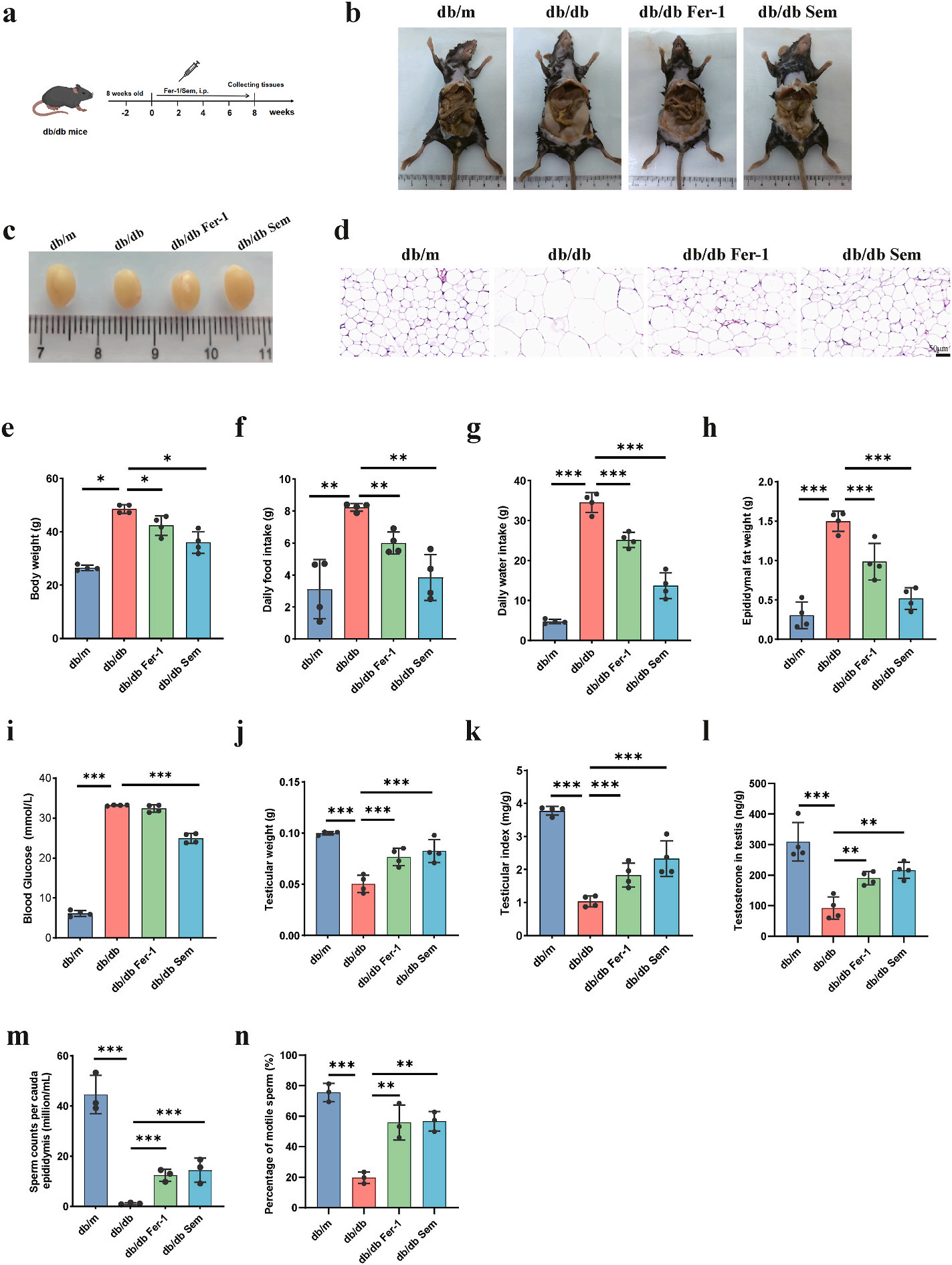
Figure 2: shows that the Semaglutide improves glucose metabolism, lipid metabolism and sperm quality in db/db mice. (a) The flowchart of the animal experiments. (b–c) Representative photographs of mouse and testis in each group. (d) Hematoxylin and eosin staining of epididymal fat. Scale bar: 50m. € Body weight. (f) Daily food intake. (g) Daily water intake. (h) Blood glucose. (i) Epididymal fat weight. (j) Testicular weight. (k) Testicular index. (l)Testosterone in testis. (m) Sperm counts per cauda epididymis. (n) Percentage of motile sperm.
Mechanism of action by which semaglutide works in improving testicular functions in diabetes : Firstly, researchers detected the lipid peroxidation marker 4-HNE and the system Xc-marker SLC7A11 by immunofluorescence in the testicular tissue. The expression of 4-HNE was significantly increased in the model group, while SLC7A11 showed a decreasing trend. After intervention with semaglutide and Fer-1, both markers were reversed The researchers findings indicated that semaglutide effectively counters diabetes-induced testicular damage by inhibiting ferroptosis. Previous studies have demonstrated semaglutide’s potential in ameliorating complications associated with metabolic disorders . However, research specifically targeting diabetes-induced testicular damage has been limited. Ferroptosis, a regulated cell death mechanism, plays a critical role in diabetes-related testicular dysfunction. It involves the accumulation of lipid peroxides and iron-dependent reactive oxygen species (ROS), leading to cell death. Researchers highlighted after a thorough research that semaglutide mitigates testicular damage by improving glucose and lipid metabolism and inhibiting ferroptosis pathway . The diabetes-induced decrease in the TM-3 cell line’s vitality, increased lipid peroxidation, ROS levels, ferrous ions, and mitochondrial membrane potential damage were all improved by semaglutide and ferrostatin-1 intervention. These results confirm that semaglutide and Fer-1 can exert their antagonistic effects on ferroptosis by antioxidant activity and the clearance of lipid peroxidation.
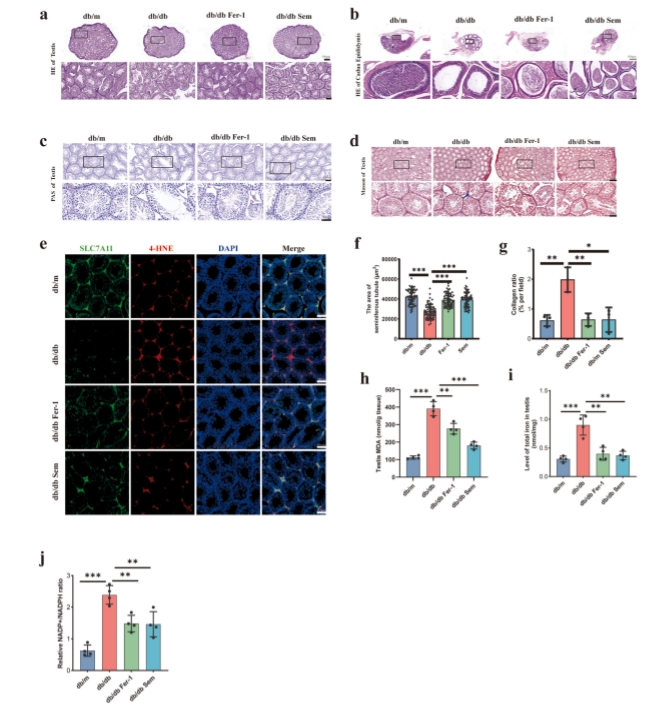
Figure 3 : shows Semaglutide ameliorates testicular dysfunction in diabetic mice by inhibiting ferroptosis.
Therapeutic potential : Researchers intervened TM-3 cells with high glucose, high lipid, and high glucose and high lipid separately. The cell viability of the HG-PA group decreased more significantly . Since disturbances in glucose and lipid metabolism can be observed in diabetic patients and mouse models, they conducted further research using the high glucose and high lipid cell model. After intervention with semaglutide and Fer-1 in the high glucose and high lipid cell model, cell vitality was partially restored . Semaglutide’s antioxidative and anti-inflammatory properties make it a promising therapeutic approach for preserving testicular function in diabetic patients. By reducing iron content and inhibiting the expression of ferroptosis markers in testicular tissue, semaglutide demonstrates significant potential in countering the adverse effects of diabetes on male reproductive health. Treatment with semaglutide (Sem) and the ferroptosis inhibitor ferrostatin-1 (Fer-1) lessens testicular destruction by refining lipid peroxidation and ferroptosis indicators. In the TM-3 cell line, semaglutide and Fer-1 lessen diabetes-induced declines in cell vitality, lipid peroxidation, reactive oxygen species (ROS), ferrous ions, and mitochondrial membrane potential damage. Scientific statistics expose that diabetes disturbs gonadotropin and testosterone levels, impairing lipid metabolism and sperm superiority. Testicular analysis from diabetic patients demonstrations less Leydig cells and higher ferroptosis markers. In diabetic mice, semaglutide and Fer-1 improved glucose and lipid metabolism, testosterone levels, and sperm quality while dipping ferroptosis indicators and improving testicular assembly. These discoveries suggest semaglutide’s potential as a management for diabetes-related testicular dysfunction by targeting the ferroptosis alleyway. Immunofluorescence detection revealed increased expression of ferroptosis markers in testes of diabetes mice, which were reversed by treatment with semaglutide. In diabetic TM-3 cells, cell viability was reduced, accompanied by abnormalities in lipid droplets, reactive oxygen species, ferrous ions, and mitochondrial membrane potential, all of which were improved by semaglutide and Fer-1 treatment.
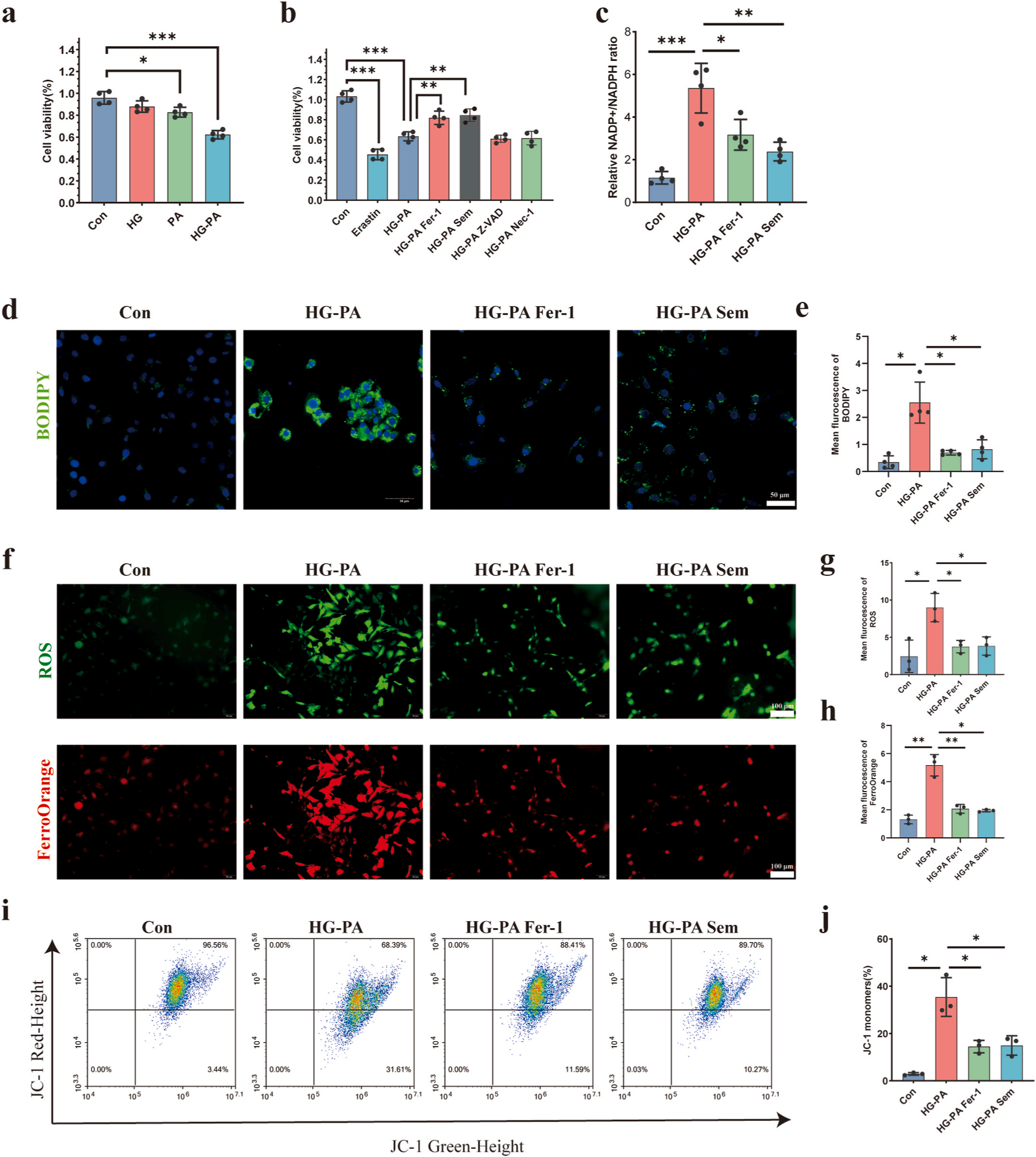
Figure 4: Shows Semaglutide attenuated HG-PA induced ferroptosis in TM-3 cells. (a) The cell viability of TM-3 cells treated with HG, PA and HG-PA . (b) The cell viability of TM-3 cells treated with Erastin(GC16630), HG-PA or HG-PA combining with the Fer-1,Sem, Z-VAD (GC12861), Nec-1(GC11008). (C) Relative NADP+/NADPH ratio of TM-3 cells . (d) Representative images of lipid peroxidation (BODIPY) (GC40165)levels of TM-3 cells under fluorescence staining. The green markers represent lipid peroxidation level. Scale bar: 50 μm. € Quantification of average BODIPY fluorescence intensity . (f) Representative images of ROS and ferrous iron levels of TM-3 cells under fluorescence staining. The green markers represent ROS level. The red markers represent FerrOrange level. Scale bar: 100 μm. (g) Quantification of average ROS fluorescence intensity . (h) Quantification of average FerrOrange(GC19943) fluorescence intensity . (i) Flow cytometry analysis of JC-1 in TM-3 cells . (j) Quantification of flow cytometry analysis for JC-1.
This study highlights the therapeutic potential of semaglutide in preserving testicular function in diabetes by modulating the ferroptosis pathway. Through comprehensive clinical data analysis and experimental work in diabetic mouse models, it has been shown that Semaglutide can effectively mitigate testicular damage, offering a novel therapeutic avenue for improving male reproductive health in diabetic patients. These conclusions propose that semaglutide mbe recommended as additional welfares in managing diabetes-related reproductive dysfunction. Future research is needed to validate these results in medical settings and to elucidate the underlying mechanisms, which could cover the way for novel therapeutic strategies in diabetes care.













Comments