Omeprazole sodium |
| رقم الكتالوجGC61155 |
أوميبرازول الصوديوم (H 16868 صوديوم) ، وهو مثبط لمضخة البروتون (PPI) ، متاح لعلاج اضطرابات الجهاز الهضمي المرتبطة بالحمض
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 95510-70-6
Sample solution is provided at 25 µL, 10mM.
Omeprazole sodium (H 16868 sodium), a proton pump inhibitor (PPI), is available for treatment of acid-related gastrointestinal disorders. Omeprazole sodium shows competitive inhibition of CYP2C19 activity with a Ki of 2 to 6 μM[1]. Omeprazole sodium also inhibits growth of Gram-positive and Gram-negative bacteria[2]. Omeprazole is a potent brain penetrant neutral sphingomyelinase (N-SMase) inhibitor (exosome inhibitor)[3].
Omeprazole (H 16868) is a proton pump inhibitor used in the treatment of dyspepsia, peptic ulcer disease, gastroesophageal reflux disease, laryngopharyngeal reflux, and Zollinger-Ellison syndrome. Omeprazole (H 16868) virtually eliminated intragastric acidity in all patients: the median 24 hour intragastric pH rose from 1.4 to 5.3 and the mean hourly hydrogen ion activity fell from 38.50 to 1.95 mmol(mEq)/1 (p less than 0.001). This inhibition of 24 hour intragastric acidity is more profound than that previously reported with either cimetidine 1 g daily or ranitidine 300 mg daily[1]. The pharmacokinetics of omeprazole were studied in a group of healthy male subjects after single and repeated oral doses of 30 and 60 mg. Absorption of Omeprazole (H 16868) from its enteric-coated formulation was unpredictable. There was a highly significant increase in the area under the plasma concentration time curve (AUC) after repeated dosing. Omeprazole (H 16868) increases its own relative availability following repeated dosing. This may be due to inhibition of gastric acid secretion by omeprazole which is an acid-labile compound[2].Omeprazole sodium is a potent brain penetrant neutral sphingomyelinase (N-SMase) inhibitor (exosome inhibitor)[3].
[1]. Li XQ, et al. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004 Aug;32(8):821-7. [2]. Jonkers D, et al. Omeprazole inhibits growth of gram-positive and gram-negative bacteria including Helicobacter pylori in vitro. J Antimicrob Chemother. 1996 Jan;37(1):145-50. [3]. Huarui Zhang, et al. Advances in the discovery of exosome inhibitors in cancer. J Enzyme Inhib Med Chem. 2020 Dec;35(1):1322-1330.
| Cas No. | 95510-70-6 | SDF | |
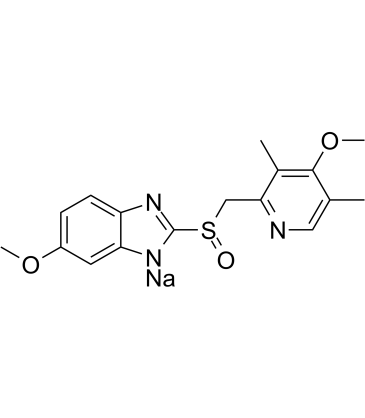
| Canonical SMILES | O=S(C1=NC2=CC=C(OC)C=C2N1[Na])CC3=NC=C(C)C(OC)=C3C | ||
| Formula | C17H18N3NaO3S | M.Wt | 367.4 |
| الذوبان | DMSO: 250 mg/mL (680.46 mM) | Storage | Store at -20°C |
| General tips | Please select the appropriate solvent to prepare the stock solution according to the
solubility of the product in different solvents; once the solution is prepared, please store it in
separate packages to avoid product failure caused by repeated freezing and thawing.Storage method
and period of the stock solution: When stored at -80°C, please use it within 6 months; when stored
at -20°C, please use it within 1 month. To increase solubility, heat the tube to 37°C and then oscillate in an ultrasonic bath for some time. |
||
| Shipping Condition | Evaluation sample solution: shipped with blue ice. All other sizes available: with RT, or with Blue Ice upon request. | ||
| Prepare stock solution | |||

|
1 mg | 5 mg | 10 mg |
| 1 mM | 2.7218 mL | 13.6091 mL | 27.2183 mL |
| 5 mM | 0.5444 mL | 2.7218 mL | 5.4437 mL |
| 10 mM | 0.2722 mL | 1.3609 mL | 2.7218 mL |
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
 g
g
 μL
μL

Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
3. All of the above co-solvents are available for purchase on the GlpBio website.
Quality Control & SDS
- View current batch:
- Purity: >98.00%
- COA (Certificate Of Analysis)
- SDS (Safety Data Sheet)
- Datasheet
Average Rating: 5 (Based on Reviews and 4 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *



















