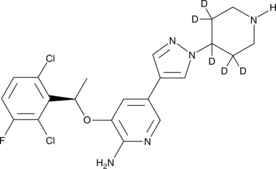
(R)-Crizotinib-d5 |
| رقم الكتالوجGC46343 |
An internal standard for the quantification of (R)-crizotinib
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 1395950-84-1
Sample solution is provided at 25 µL, 10mM.
(R)-Crizotinib-d5 is intended for use as an internal standard for the quantification of (R)-crizotinib by GC- or LC-MS. (R)-Crizotinib is a derivative of aminopyridine that acts as a potent, orally bioavailable, ATP-competitive small-molecule dual inhibitor of c-MET (IC50 = 8 nM) and ALK (IC50 = 20 nM) receptor tyrosine kinases.1 (R)-Crizotinib shows antitumor efficacy, including cytoreductive antitumor activity, in multiple tumor models implanted in athymic mice that express activated c-MET or ALK fusion proteins (IC50s = 5-20 nM).1,2
1.Cui, J.J., Tran-DubÉ, M., Shen, H., et al.Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK)J. Med. Chem.54(18)6342-6363(2011) 2.Tanizaki, J., Okamoto, I., Okamoto, K., et al.MET tyrosine kinase inhibitor crizotinib (PF-02341066) shows differential antitumor effects in non-small cell lung cancer according to MET alterationsJ.Thorac.Oncol.6(10)1624-1631(2011)
Average Rating: 5 (Based on Reviews and 28 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















