Rezivertinib (Synonyms: BPI-7711) |
| رقم الكتالوجGC62341 |
Rezivertinib (BPI-7711) هو مثبط الجيل الثالث من مثبطات EGFR التيروزين كيناز (TKI) النشط عن طريق الفم ، وانتقائي للغاية ولا رجعة فيهيُظهر Rezivertinib فاعلية عالية ضد التنشيط الشائع EGFR وطفرات المقاومة T790MRezivertinib لديه اختراق ممتاز للجهاز العصبي المركزي (CNS) وله نشاط مضاد للأورام
Products are for research use only. Not for human use. We do not sell to patients.
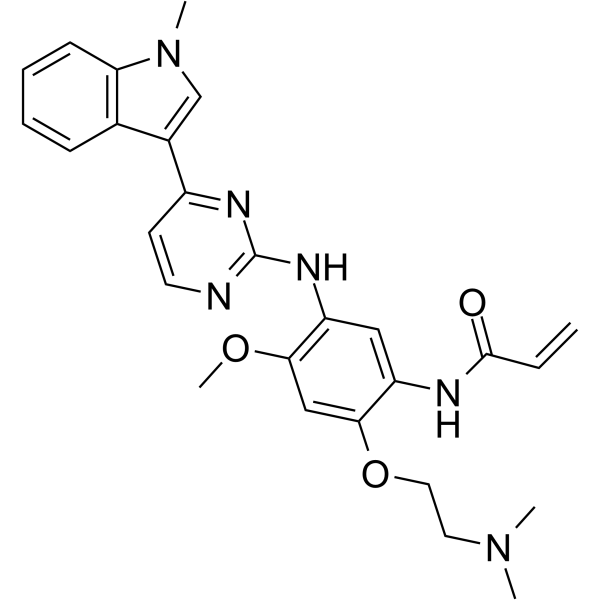
Cas No.: 1835667-12-3
Sample solution is provided at 25 µL, 10mM.
Rezivertinib (BPI-7711) is an orally active, highly selective and irreversible third-generation EGFR tyrosine kinase inhibitor (TKI). Rezivertinib exhibits high potency against the common activation EGFR and the resistance T790M mutations. Rezivertinib has excellent central nervous system (CNS) penetration and has antitumor activity[1][2].
Rezivertinib (BPI-7711) selectively inhibits cellular proliferation of EGFR mutations in cell lines: GI50 13.3 nM (PC9, del19), 6.8 nM (HCC827, L858R), 22 nM (NCI-H1975, del19/T790M) and > 1000 nM (A431, EGFR WT)[1].
Rezivertinib (BPI-7711; 6.25-25 mg/kg/day; orally; 14 days) shows significant tumor regression[2]. Rezivertinib (12.5 mg/kg/day; orally; 14 days) survives an average of 112% longer in H1975-luc human NSCLC mice model[2]. Rezivertinib (50 mg/kg/day; orally) has anti-tumor efficacy correlated to improved average overall survival of the animals of 115% (28 days vs. 13 days)[2].
[1]. Misako Nagasaka, et al. Beyond Osimertinib: The Development of Third-Generation EGFR Tyrosine Kinase Inhibitors For Advanced EGFR+ NSCLC. J Thorac Oncol. 2021 May;16(5):740-763.
[2]. Victoria L. Wilde, et al. Preclinical evidence of BPL-7711 activity in Egfr-mutant non-smallcell lung cancer ( NSCLC ) in orthotopically implanted human tumorxenografts in the lung and brain.
Average Rating: 5 (Based on Reviews and 15 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















