TFLLR-NH2 |
| رقم الكتالوجGC17444 |
TFLLR-NH2 هو ناهض PAR1 انتقائي مع EC50 من 1.9 ميكرومتر
Products are for research use only. Not for human use. We do not sell to patients.
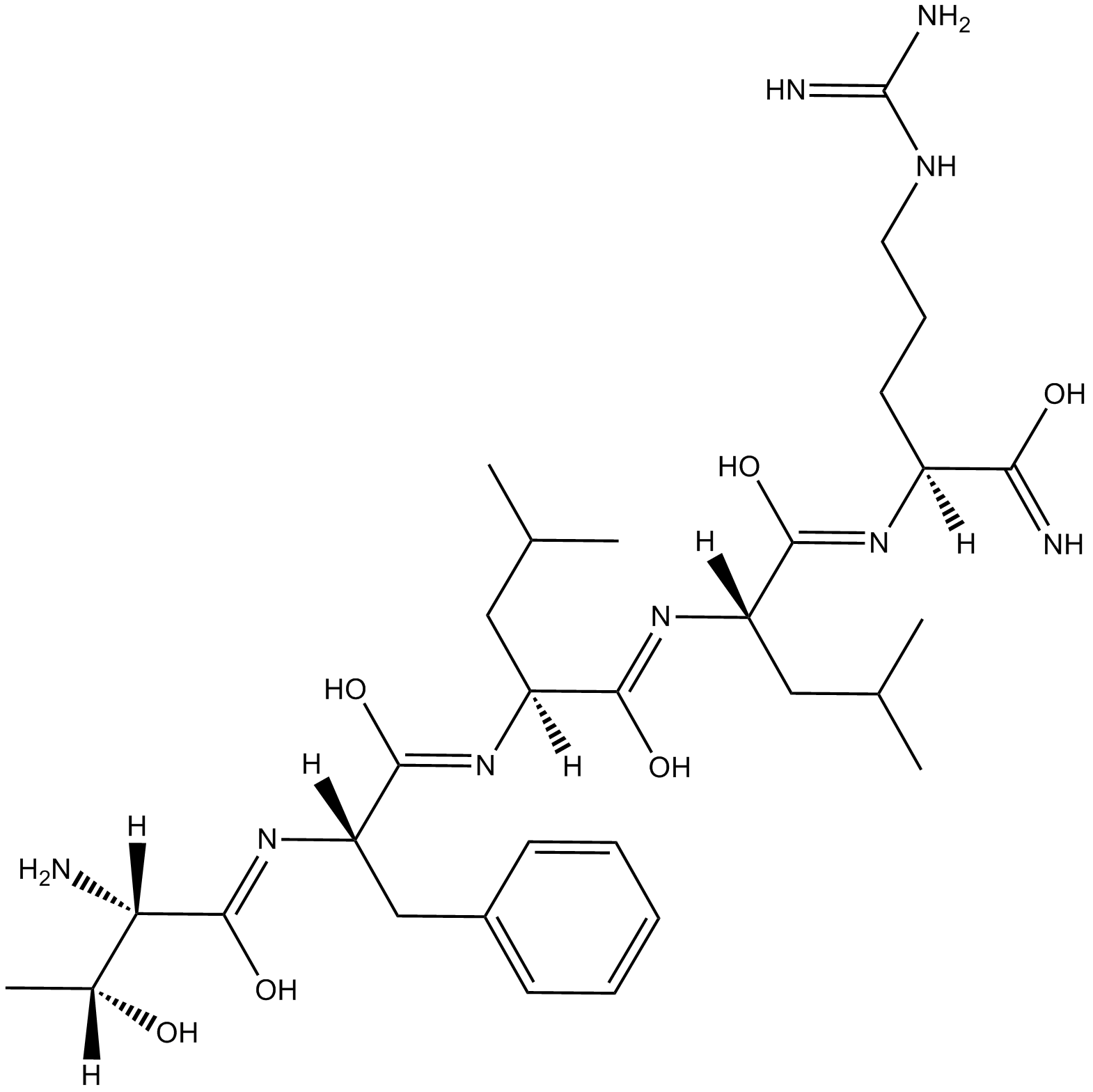
Cas No.: 197794-83-5
Sample solution is provided at 25 µL, 10mM.
TFLLR-NH2 is a selective PAR1 agonist with an EC50 of 1.9 μM.
PAR1 agonists stimulate concentration-dependent increases in [Ca2+]i and in the proportions of neurones. The maximal increase in [Ca2+]i above basal is detected in response to 10 μm TF-NH2(peak 196.5±20.4 nM, n=25) when 50–80% of identified neurones responded[1]. SW620 cells cultured in the supernatant of TFLLR-NH2-activated platelets upregulate E-cadherin expression and downregulate the vimentin expression. In the in vitro platelet culture system, a TFLLR-NH2 dose-dependent increase of secreted TGF-β1 is detected in the supernatant[2].
Injection of TF-NH2 into the rat paw stimulates a marked and sustained oedema. An NK1R antagonist and ablation of sensory nerves with capsaicin inhibit oedema by 44% at 1 h and completely by 5 h. In wild-type but not PAR1−/− mice, TF-NH2 stimulates Evans blue extravasation in the bladder, oesophagus, stomach, intestine and pancreas by 2–8 fold. Extravasation in the bladder, oesophagus and stomach is abolished by an NK1R antagonist[1]. TFp-NH2 produces notable contraction at 3-50 μM and relaxation at 0.3-50 μM, in the absence of apamin. The concentration-response curve for TFp-NH2-induced contraction is remarkably shifted left, when the TFp-NH2-induced relaxation is blocked by apamin at 0.1 μM[3].
References:
[1]. de Garavilla L, et al. Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism. Br J Pharmacol. 2001 Aug;133(7):975-87.
[2]. Kawabata A, et al. Characterization of the protease-activated receptor-1-mediated contraction and relaxation in the rat duodenal smooth muscle.
[3]. Jia Y, et al. Activation of platelet protease-activated receptor-1 induces epithelial-mesenchymal transition and chemotaxis of colon cancer cell line SW620. Oncol Rep. 2015 Jun;33(6):2681-8.
Animal experiment: | Mice: Mice are anaesthetized with isofluorane, and saline or TF-NH2 (3?μmol/kg in 25?μL physiological saline) is injected into the lateral tail vein. Evans blue (33.3mg/kg in 50?μL saline) is co-injected with the peptide. Mice are perfused transcardially at 10?min after administration of TF-NH2 with physiological saline containing 20?u/mL heparin at a pressure of 80-100?mmHg for 2-3?min. Excised tissues are incubated in 1?mL of formamide for 48?h, and Evans blue content is measured spectrophotometrically at 650?nm[1]. |
References: [1]. de Garavilla L, et al. Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism. Br J Pharmacol. 2001 Aug;133(7):975-87. | |
| Cas No. | 197794-83-5 | SDF | |
| Chemical Name | (S,Z)-2-((Z)-((S)-2-((Z)-((2S,3R)-2-amino-1,3-dihydroxybutylidene)amino)-1-hydroxy-3-phenylpropylidene)amino)-N-((S,Z)-1-(((S)-5-guanidino-1-hydroxy-1-iminopentan-2-yl)imino)-1-hydroxy-4-methylpentan-2-yl)-4-methylpentanimidic acid | ||
| Canonical SMILES | CC(C[C@@](/N=C(O)/[C@](/N=C(O)/[C@](/N=C(O)/[C@](N)([H])[C@@](O)([H])C)([H])CC1=CC=CC=C1)([H])CC(C)C)([H])/C(O)=N/[C@@](C(O)=N)([H])CCCNC(N)=N)C | ||
| Formula | C31H53N9O6 | M.Wt | 647.82 |
| الذوبان | Soluble to 1 mg/ml in 20% acetonitrile / sterile water | Storage | Desiccate at -20°C |
| General tips | Please select the appropriate solvent to prepare the stock solution according to the
solubility of the product in different solvents; once the solution is prepared, please store it in
separate packages to avoid product failure caused by repeated freezing and thawing.Storage method
and period of the stock solution: When stored at -80°C, please use it within 6 months; when stored
at -20°C, please use it within 1 month. To increase solubility, heat the tube to 37°C and then oscillate in an ultrasonic bath for some time. |
||
| Shipping Condition | Evaluation sample solution: shipped with blue ice. All other sizes available: with RT, or with Blue Ice upon request. | ||
| Prepare stock solution | |||

|
1 mg | 5 mg | 10 mg |
| 1 mM | 1.5436 mL | 7.7182 mL | 15.4364 mL |
| 5 mM | 0.3087 mL | 1.5436 mL | 3.0873 mL |
| 10 mM | 0.1544 mL | 0.7718 mL | 1.5436 mL |
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
 g
g
 μL
μL

Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
3. All of the above co-solvents are available for purchase on the GlpBio website.
Quality Control & SDS
- View current batch:
- Purity: >98.00%
- COA (Certificate Of Analysis)
- SDS (Safety Data Sheet)
- Datasheet
Average Rating: 5 (Based on Reviews and 38 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *



















