Brefeldin A (Synonyms: Ascotoxin, BFA, Cyanein, Decumbin, Nectrolide, NSC 56310, NSC 89671, NSC 107456, NSC 244390, Synergisidin) |
| Catalog No.GC17683 |
Brefeldin A (BFA) is a fungal macrocyclic lactone and a potent, reversible inhibitor of intracellular vesicle formation and protein trafficking between the endoplasmic reticulum (ER) and the Golgi apparatus.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 20350-15-6
Sample solution is provided at 25 µL, 10mM.
Brefeldin A (BFA) is a fungal macrocyclic lactone and a potent, reversible inhibitor of intracellular vesicle formation and protein trafficking between the endoplasmic reticulum (ER) and the Golgi apparatus[1][2].Brefeldin A is an ATPase inhibitor with IC50 value of 0.2 µM[9].Brefeldin A and its analogs are promising inhibitors in drug development due to a number of key features such as apoptosis?inducing properties as well as antitumor, antifungal, and antiviral effects [3,6].Brefeldin A is a CRISPR/Cas9 activator. Brefeldin A inhibits HSV-1 and has anti-cancer activity[4].
Perturbation of ER-Golgi trafficking by brefeldin A (BFA) treatment attenuated nucleotide-binding oligomerization domain-like receptor family, pyrin-domain-containing 3 (NLRP3) inflammasome activation in mouse bone marrow-derived macrophages (BMDMs) [5].ADP-ribosylation of BARS is mediated by formation of a conjugate between Brefeldin A and ADPR. BARS shows BAC binding when incubated with the medium from the brefeldin A-treated CD38+ HeLa cells[3].Brefeldin A induces anchorage-independent cell death in MDA-MB-231 breast cancer cells with an EC50 of 0.016 µg/mL, inhibits the formation of MDA-MB-231 colonies in 3D and 2D cultures and inhibits the migration and MMP 9 activity of MDA-MB-231[2].
In tumor-bearing mice, M-brefeldin A can prolong blood circulation, improve tumor accumulation ability, and show effective inhibition of tumor growth, M-brefeldin A 10 mg/kg group showed effective antitumor effect and significantly delayed tumor progression, while M-brefeldin A 5 mg/kg mice did not show significant inhibitory effect[7]. Mice were treated with the Golgi blocker Brefeldin A. Since most cytokines are processed and secreted via the classical secretion pathway through the Golgi, brefeldin A blocks cytokine secretion, leading to their accumulation within immune cells, which are eventually detected by flow cytometry. Thus, treatment of mice with brefeldin A allows in situ assessment of cytokine production without the use of reporter mice [8].
References:
[1]: Orci L, Tagaya M, et,al. Brefeldin A, a drug that blocks secretion, prevents the assembly of non-clathrin-coated buds on Golgi cisternae. Cell. 1991 Mar 22;64(6):1183-95. doi: 10.1016/0092-8674(91)90273-2. PMID: 2004424.
[2]: Tseng CN, Hong YR, et,al. Brefeldin A reduces anchorage-independent survival, cancer stem cell potential and migration of MDA-MB-231 human breast cancer cells. Molecules. 2014 Oct 29;19(11):17464-77. doi: 10.3390/molecules191117464. PMID: 25356567; PMCID: PMC6271931.
[3]: Wang J, Fang Y, et,al. Erythroleukemia cells acquire an alternative mitophagy capability. Sci Rep. 2016 Apr 19;6:24641. doi: 10.1038/srep24641. PMID: 27091640; PMCID: PMC4835698.
[4]: Yu C, Liu Y, et,al. Small molecules enhance CRISPR genome editing in pluripotent stem cells. Cell Stem Cell. 2015 Feb 5;16(2):142-7. doi: 10.1016/j.stem.2015.01.003. PMID: 25658371; PMCID: PMC4461869.
[5]: Hong S, Hwang I, et,al. Brefeldin A-sensitive ER-Golgi vesicle trafficking contributes to NLRP3-dependent caspase-1 activation. FASEB J. 2019 Mar;33(3):4547-4558. doi: 10.1096/fj.201801585R. Epub 2018 Dec 28. PMID: 30592629.
[6]: Paek SM. Recent Synthesis and Discovery of Brefeldin A Analogs. Mar Drugs. 2018 Apr 18;16(4):133. doi: 10.3390/md16040133. PMID: 29670019; PMCID: PMC5923420.
[7]: Zhang JM, Jiang YY, et,al. Brefeldin A delivery nanomicelles in hepatocellular carcinoma therapy: Characterization, cytotoxic evaluation in vitro, and antitumor efficiency in vivo. Pharmacol Res. 2021 Oct;172:105800. doi: 10.1016/j.phrs.2021.105800. Epub 2021 Aug 4. PMID: 34363949.
[8]: Kovacs SB, Oh C, et,al. Evaluating cytokine production by flow cytometry using brefeldin A in mice. STAR Protoc. 2020 Dec 30;2(1):100244. doi: 10.1016/j.xpro.2020.100244. PMID: 33458706; PMCID: PMC7797915.
[9]: Wierzbicki PM, Kogut-Wierzbicka M, et,al. Protein and siRNA delivery by transportan and transportan 10 into colorectal cancer cell lines. Folia Histochem Cytobiol. 2014;52(4):270-80. doi: 10.5603/FHC.a2014.0035. Epub 2014 Dec 16. PMID: 25511292.
| Cell experiment [1]: | |
|
Cell lines |
Mouse primary bone marrow-derived macrophages (BMDMs) from the femurs of C57BL/6 or Nlrp-/- mice |
|
Preparation Method |
Quantification of IL-6 and IL-1β in the culture supernatants of mouse BMDMs untreated (Unt) or treated with LPS in the presence of Golgi-Plug, Brefeldin A (2 or 5 ug/ ml), or MCC950 for 3 h followed by ATP treatment |
|
Reaction Conditions |
2 or 5 ug/ ml Brefeldin A for 3 h |
|
Applications |
Inhibition of vesicle trafficking by Brefeldin A between ER and golgi attenuates IL-1β production from BMDMs upon stimulation with NLRP3 agonists |
| Animal experiment [2]: | |
|
Animal models |
Female BALB/ C mice (20 ± 2 g, 5-6 weeks) |
|
Preparation Method |
HepG2 tumor-bearing nude mice were injected with M- Brefeldin A daily for 14 days( intravenous injection) |
|
Dosage form |
5 mg/kg and 10 mg/kg Brefeldin A for 14 days |
|
Applications |
M- Brefeldin A 10 mg/kg group showed effective antitumor effect and significantly delayed tumor progression, while M- Brefeldin A 5 mg/kg mice did not show significant inhibitory effect. |
|
References: [1]. Hong S, Hwang I, et,al. Brefeldin A-sensitive ER-Golgi vesicle trafficking contributes to NLRP3-dependent caspase-1 activation. FASEB J. 2019 Mar;33(3):4547-4558. doi: 10.1096/fj.201801585R. Epub 2018 Dec 28. PMID: 30592629. | |
| Cas No. | 20350-15-6 | SDF | |
| Synonyms | Ascotoxin, BFA, Cyanein, Decumbin, Nectrolide, NSC 56310, NSC 89671, NSC 107456, NSC 244390, Synergisidin | ||
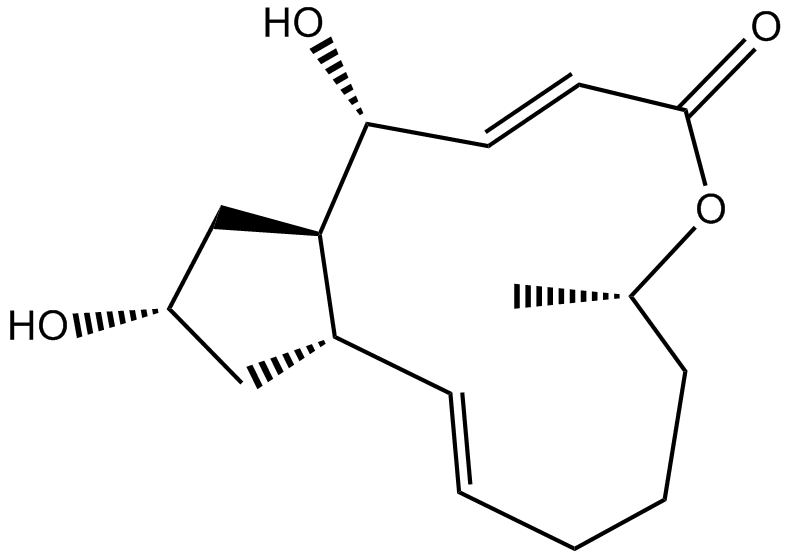
| Chemical Name | (1S,2E,7S,10E,12R,13R,15S)-12,15-dihydroxy-7-methyl-8-oxabicyclo[11.3.0]hexadeca-2,10-dien-9-one | ||
| Canonical SMILES | CC1CCCC=CC2CC(CC2C(C=CC(=O)O1)O)O | ||
| Formula | C16H24O4 | M.Wt | 280.36 |
| Solubility | ≥ 4.67mg/mL in DMSO | Storage | Store at -20°C |
| General tips | Please select the appropriate solvent to prepare the stock solution according to the
solubility of the product in different solvents; once the solution is prepared, please store it in
separate packages to avoid product failure caused by repeated freezing and thawing.Storage method
and period of the stock solution: When stored at -80°C, please use it within 6 months; when stored
at -20°C, please use it within 1 month. To increase solubility, heat the tube to 37°C and then oscillate in an ultrasonic bath for some time. |
||
| Shipping Condition | Evaluation sample solution: shipped with blue ice. All other sizes available: with RT, or with Blue Ice upon request. | ||
| Prepare stock solution | |||

|
1 mg | 5 mg | 10 mg |
| 1 mM | 3.5668 mL | 17.8342 mL | 35.6684 mL |
| 5 mM | 0.7134 mL | 3.5668 mL | 7.1337 mL |
| 10 mM | 0.3567 mL | 1.7834 mL | 3.5668 mL |
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
 g
g
 μL
μL

Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
3. All of the above co-solvents are available for purchase on the GlpBio website.
Quality Control & SDS
- View current batch:
- Purity: >99.50%
- COA (Certificate Of Analysis)
- SDS (Safety Data Sheet)
- Datasheet
Average Rating: 5 (Based on Reviews and 5 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




