α-Angelica lactone |
| Katalog-Nr.GC61980 |
α-Angelikalacton ist ein natÜrlich vorkommendes Antikarzinogen und ein vinyloges Nucleophil. α-Angelica-Lacton kann die chiralen δ-Amino γ,γ-disubstituierten Butenolid-Carbonylderivate ergeben und ein selektivrophiles Abfangen am γ-Kohlenstoff aufweisen. α-Angelikalacton Übt starke chemoprotektive Wirkungen durch selektive VerstÄrkung der Entgiftungsenzyme Glutathion-S-thansferase (GST) und UDP-Glucononosyltransferase (UGT) aus.
Products are for research use only. Not for human use. We do not sell to patients.
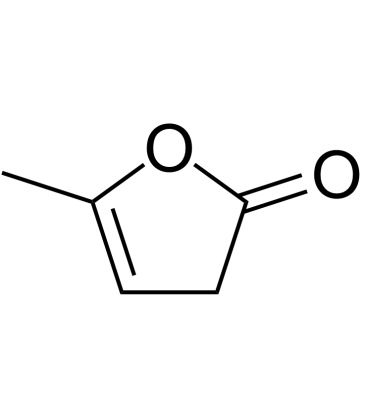
Cas No.: 591-12-8
Sample solution is provided at 25 µL, 10mM.
α-Angelica lactone is a naturally occurring anticarcinogen and an vinylogous nucleophile. α-Angelica lactone can give the chiral δ-amino γ,γ-disubstituted butenolide carbonyl derivatives and exhibitselectrophilic trapping at the γ-carbon. α-Angelica lactone exerts strong chemoprotective effects by selective enhancement of glutathione-S-thansferase (GST) and UDP-glucononosyltransferase (UGT) detoxification enzymes[1][2][3][4].
[1]. W A Nijhoff, et al. Quantification of Induction of Rat Oesophageal, Gastric and Pancreatic Glutathione and Glutathione S-transferases by Dietary Anticarcinogens. Carcinogenesis. 1994 Sep;15(9):1769-72.
[2]. E M J van der Logt, et al. Induction of Rat Hepatic and Intestinal UDP-glucuronosyltransferases by Naturally Occurring Dietary Anticarcinogens. Carcinogenesis. 2003 Oct;24(10):1651-6.
[3]. Lin Zhou, et al. Catalytic Asymmetric Vinylogous Mannich-type (AVM) Reaction of Nonactivated α-Angelica Lactone. Org Lett. 2011 Jun 17;13(12):3056-9.
[4]. Jessica A Griswold, et al. Diastereoselective Organocatalytic Addition of α-Angelica Lactone to β-Halo-α-ketoesters. J Org Chem. 2017 Feb 17;82(4):2276-2280.
Average Rating: 5 (Based on Reviews and 22 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















