BCI (Synonyms: (E)-BCI) |
| Katalog-Nr.GC38646 |
BCI, als selektiver Dual-spezifischer Phosphatase-6 (DUSP6)-Inhibitor, kann das Tumorwachstum und die Entzündung von Makrophagen hemmen.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 1245792-51-1
Sample solution is provided at 25 µL, 10mM.
BCI, als selektiver Dual-spezifischer Phosphatase-6 (DUSP6)-Inhibitor, kann das Tumorwachstum und die Entzündung von Makrophagen hemmen.[1].
In vitro zeigte BCI bei niedrigen Dosen von ≤2 μM und ≤4 μM keine zytotoxischen Effekte auf RAW264.7-Zellen bzw. BMMs. Und bei Konzentrationen von ≤4 μM hatte BCI keinen offensichtlichen Einfluss auf den Zellzyklus oder die Apoptose in BMMs.[1] In in vitro Experimenten wurde gezeigt, dass die Behandlung mit 1 μM BCI die Osteoklastogenese durch Hemmung von DUSP6 verstärkte. Darüber hinaus erhöhte BCI die Expression von osteoklastenbezogenen Genen wie NFATC1, C-fos, ACP5 und DC-STAMP.[2] In in vitro Wirksamkeitstests wurde gezeigt, dass die Behandlung mit 4 μM BCI den Anteil an Zellen, die gespaltenes Caspase-3 exprimieren, deutlich erhöhte, und 4 μM BCI verursachte bereits eine umfangreiche Zytotoxizität in KELLY- und IMR-32-Zellen, wobei nur eine Minderheit der LAN-1- und SK-N-AS-Zellen überlebte.[3] In vitro beeinflusste 1 μM BCI nicht die Gesamt-NCC- und NCC-Oberflächenexpression sowie die ERK1/2-Phosphorylierung. Die Behandlung mit 5 μM BCI kann die ERK1/2-Phosphorylierung deutlich erhöhen und die Gesamt-NCC- und NCC-Oberflächenexpression verringern.[5]
In vivo wurden Mäuse fünf aufeinanderfolgende Tage pro Woche mit 10 mg/kg BCI intraperitoneal behandelt, was die AKT-Aktivierung unterdrückte und die Tumorbildung verhinderte.[4] In in vivo Tests wurde gezeigt, dass die Behandlung mit 50, 100 und 200 mg/kg BCI oral im CPDM-Tiermodell die Anzahl der pNrf2-positiven Zellen im parodontalen Gewebe deutlich erhöhte und den Verlust des Alveolarknochens milderte.[6]
References:
[1] Cai C, et al. BCI Suppresses RANKL-Mediated Osteoclastogenesis and Alleviates Ovariectomy-Induced Bone Loss. Front Pharmacol. 2021 Nov 1;12:772540.
[2] Zhang B, et al. DUSP6 expression is associated with osteoporosis through the regulation of osteoclast differentiation via ERK2/Smad2 signaling. Cell Death Dis. 2021 Sep 2;12(9):825.
[3] Thompson EM, et al. The cytotoxic action of BCI is not dependent on its stated DUSP1 or DUSP6 targets in neuroblastoma cells. FEBS Open Bio. 2022 Jul;12(7):1388-1405.
[4] Duan S, et al. Loss of FBXO31-mediated degradation of DUSP6 dysregulates ERK and PI3K-AKT signaling and promotes prostate tumorigenesis. Cell Rep. 2021 Oct 19;37(3):109870.
[5] Feng X, et al. Aldosterone modulates thiazide-sensitive sodium chloride cotransporter abundance via DUSP6-mediated ERK1/2 signaling pathway. Am J Physiol Renal Physiol. 2015 May 15;308(10):F1119-27.
[6] Zhu C, et al. The therapeutic role of baicalein in combating experimental periodontitis with diabetes via Nrf2 antioxidant signaling pathway. J Periodontal Res. 2020 Jun;55(3):381-391.
| Cell experiment [1]: | |
Cell lines | MPNST cells |
Preparation Method | MPNST cells were starved overnight, incubated with BCI (2 uM) for 60 mins then stimulated with DMEM and 10% FBS for 1 hr. Immunoblot analysis of TP53, p-RB and p-ATM and PARP cleavage and CC3 in ST8814 and S462.TY MPNST cells 24h after treatment with BCI (2 uM). |
Reaction Conditions | 2 uM; 60 mins |
Applications | After 1 hr, p-ERK, p-JNK, p-c-jun and total c-jun were elevated in the BCI-treated MPNST cell lines ST8814 and S462.TY but did not change in iHSC-1λ. Within 24 hours, BCI decreased total PARP and increased cleaved PARP and CC3, indicative of apoptotic cell death in NF1 deficient ST8814 and S462.TY cells. |
| Animal experiment [2]: | |
Animal models | Female C57BL/6 mice (8-weeks old) |
Dosage form | 15 mg/kg or 30 mg/kg; i.p. |
Preparation method | Low- or high-concentration (15 mg/kg or 30 mg/kg) BCI was injected intraperitoneally for 8 weeks, and bone loss was evaluated by micro-CT. |
Applications | Bone loss was prevented in both the low- and high-concentration BCI groups. Moreover, quantitative results indicated obvious increases in bone volume/total tissue volume (BV/TV), trabecular number (Tb.N), bone mineral density (BMD), and bone surface density (BS/TV) in both BCI-treated groups relative to the OVX group. |
References: | |
| Cas No. | 1245792-51-1 | SDF | |
| Überlieferungen | (E)-BCI | ||
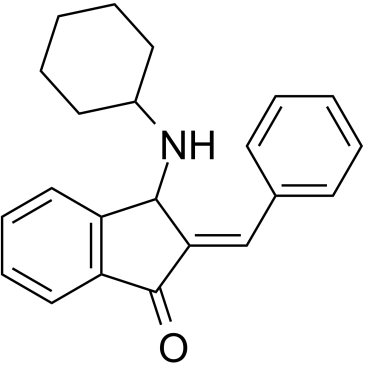
| Canonical SMILES | O=C1/C(C(NC2CCCCC2)C3=C1C=CC=C3)=C/C4=CC=CC=C4 | ||
| Formula | C22H23NO | M.Wt | 317.42 |
| Löslichkeit | DMSO: 125 mg/mL (393.80 mM) | Storage | Store at -20°C |
| General tips | Please select the appropriate solvent to prepare the stock solution according to the
solubility of the product in different solvents; once the solution is prepared, please store it in
separate packages to avoid product failure caused by repeated freezing and thawing.Storage method
and period of the stock solution: When stored at -80°C, please use it within 6 months; when stored
at -20°C, please use it within 1 month. To increase solubility, heat the tube to 37°C and then oscillate in an ultrasonic bath for some time. |
||
| Shipping Condition | Evaluation sample solution: shipped with blue ice. All other sizes available: with RT, or with Blue Ice upon request. | ||
| Prepare stock solution | |||

|
1 mg | 5 mg | 10 mg |
| 1 mM | 3.1504 mL | 15.752 mL | 31.504 mL |
| 5 mM | 0.6301 mL | 3.1504 mL | 6.3008 mL |
| 10 mM | 0.315 mL | 1.5752 mL | 3.1504 mL |
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
 g
g
 μL
μL

Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
3. All of the above co-solvents are available for purchase on the GlpBio website.
Quality Control & SDS
- View current batch:
- Purity: >98.00%
- COA (Certificate Of Analysis)
- SDS (Safety Data Sheet)
- Datasheet
Average Rating: 5 (Based on Reviews and 7 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *



















