Idarubicin HCl (Synonyms: 4Demethoxydaunorubicin, 4DMD, NSC 256439) |
| Katalog-Nr.GC14969 |
Idarubicin-HCl ist ein Anthracyclin-AntileukÄmie-Medikament.
Products are for research use only. Not for human use. We do not sell to patients.
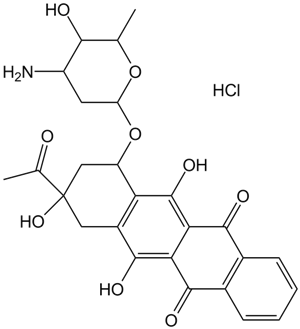
Cas No.: 57852-57-0
Sample solution is provided at 25 µL, 10mM.
Idarubicin is an inhibitor of DNA topoisomerase II [1].
Idarubicin is a synthetic anthracycline anticancer drug widely used in the treatment of acute myelogenous leukemia and some other hematological malignancies. It can be bioactivated by NADPH-cytochrome P450 reductase with resulting formation of single-strand breaks in DNA. This is the mechanism of Idarubicin ‘s antitumor effect [2].
Idarubicin is developed in an attempt to reduce the cardiotoxicity and enhance the therapeutic efficacy of the parent compound. Unlike the parent compound, Idarubicin can be given orally and has a better therapeutic index with respect to cardiotoxicity. Idarubicin has been shown to be an effective anti-leukemic agent in children and adults [3].
References:
[1] H. Dorota Halicka, M. Fevzi Ozkaynak, Oya Levendoglu-Tugal, Claudio Sandoval , Karen Seiter, Malgorzata Kajstura, Frank Traganos, Somasunadaram Jayabose, and Zbigniew Darzynkiewicz. DNA damage response as a biomarker in treatment of leukemias. Cell Cycle. 2009, 8(11): 1720–1724.
[2] Haydar Çelik and Emel Arinç. Evaluation of the Protective Effects of Quercetin, Rutin, Resveratrol, Naringenin and Trolox Against Idarubicin-Induced DNA Damage. J Pharm Pharmaceut Sci. 2010, 13(2): 231 – 241.
[3] Ching-Hon Pui, Siebold S. N. de Graaf, Lois W. Dow, John H. Rodman, William E. Evans, Bruce S. Alpert and Sharon B. Murphy. Phase I Clinical Trial of Orally Administered 4-Demethoxydaunorubicin (Idarubicin) with Pharmacokinetic and in Vitro Drug Sensitivity Testing in Children with Refractory Leukemia. Cancer Research. 1988, 48: 5348-5352.
Average Rating: 5 (Based on Reviews and 24 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















