Tebapivat |
| Katalog-Nr.GC70751 |
Tebapivat (PKR-Aktivator 2) ist ein potenter Pyruvatkinase-R (PKR)-Aktivator, extrahiert aus dem Patent WO2019035863A1, Verbindung 385.
Products are for research use only. Not for human use. We do not sell to patients.
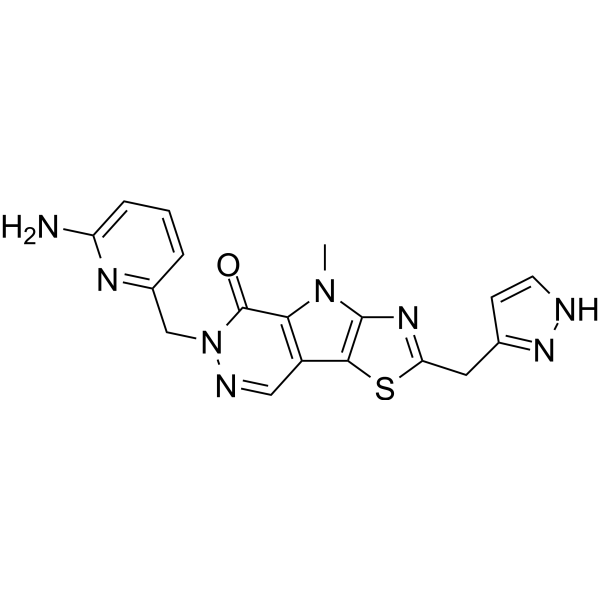
Cas No.: 2283422-04-6
Sample solution is provided at 25 µL, 10mM.
Tebapivat (compound 385) activates wild type PKR, PKR K410E or PKR 510Q with AC50 values <0.3 μΜ[1].
Pyruvate kinase deficiency (PKD) is a disease of red blood cells caused by a deficiency of pyruvate kinase R (PKR) enzyme as a result of autosomal recessive mutations of the PKLR gene. PKR activators can be beneficial to treat disorders and conditions such as but not limited to PKD, thalassemia, hereditary elliptocytosis, anemia (e.g. , congenital anemias (e.g., enzymopathies), hemolytic anemia (e.g. hereditary and/or congenital hemolytic anemia, acquired hemolytic anemia, chronic hemolytic anemia caused by phosphoglycerate kinase deficiency, anemia of chronic diseases, non-spherocytic hemolytic anemia or hereditary spherocytosis)[1].
References:
[1]. Giovanni Cianchetta, et al. Pyruvate kinase activators for use in treating blood disorders. WO2019035863A1.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















