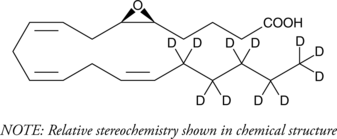
(±)5(6)-EET-d11 (Synonyms: (±)5,6-EET-d11) |
| Catalog No.GC46262 |
A neuropeptide with diverse biological activities
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: N/A
Sample solution is provided at 25 µL, 10mM.
5(6)-EET-d11 is intended for use as an internal standard for the quantification of (±)5(6)-EET by GC- or LC-MS. 5(6)-EET is a fully racemic version of the enantiomeric forms biosynthesized from arachidonic acid by cytochrome P450 enzymes.1,2 In solution, 5(6)-EET degrades into 5,6-DiHET and 5(6)-δ-lactone, which can be converted to 5(6)-DiHET and quantified by GC-MS.3 In neuroendocrine cells, such as the anterior pituitary and pancreatic islets, 5(6)-EET has been implicated in the mobilization of calcium and hormone secretion.4,5 5(6)-EET is an inhibitor of T-type voltage-gated calcium channels (Cav3) that inhibits isoforms Cav3.1, Cav3.2 (IC50 = 0.54 µM), and Cav3.3 and decreases nifedipine-resistant phenylephrine-induced vasoconstriction in isolated mouse mesenteric arteries via Cav3.2 blockade when used at a concentration of 3 µM.6 In addition, it is a substrate of COX-1 and COX-2, as measured by oxygen consumption and product formation assays when used at a concentration of 50 µM.7 (±)5(6)-EET-d11 is provided as a mixture of the free acid and lactone.
1.Chacos, N., Falck, J.R., Wixtrom, C., et al.Novel epoxides formed during the liver cytochrome P-450 oxidation of arachidonic acidBiochem. Biophys. Res. Commun.104(3)916-922(1982) 2.Oliw, E.H., Guengerich, F.P., and Oates, J.A.Oxygenation of arachidonic acid by hepatic monooxygenases. Isolation and metabolism of four epoxide intermediatesJ. Biol. Chem.257(7)3771-3781(1982) 3.Rashid, M., Manivet, P., Nishio, H., et al.Identification of the binding sites and selectivity of sarpogrelate, a novel 5-HT2 antagonist, to human 5-HT2A, 5-HT2B and 5-HT2C receptor subtypes by molecular modelingLife Sci.73(2)193-207(2003) 4.Snyder, C., Lattanzio, F., Yadagiri, P., et al.5,6-Epoxyeicosatrienoic acid mobilizes Ca2+ in anterior pituitary cellsBiochem. Biophys. Res. Commun.139(3)1188-1194(1986) 5.Falck, J.R., Manna, S., Moltz, J., et al.Epoxyeicosatrienoic acids stimulate glucagon and insulin release from isolated rat pancreatic isletsBiochem. Biophys. Res. Commun.114(2)743-749(1983) 6.Cazade, M., Bidaud, I., Hansen, P.B., et al.5,6-EET potently inhibits T-type calcium channels: Implication in the regulation of the vascular tonePflugers Arch.466(9)1759-1768(2014) 7.Rand, A.A., Barnych, B., Morisseau, C., et al.Cyclooxygenase-derived proangiogenic metabolites of epoxyeicosatrienoic acidsProc. Natl. Acad. Sci. USA114(17)4370-4375(2017)
Average Rating: 5 (Based on Reviews and 31 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *



















