Agomelatine
A Novel Therapeutic Agent for Rheumatoid Arthritis, Agomelatine (AOM) is a promising pharmacological agent that has shown significant therapeutic potential in combating rheumatoid arthritis (RA), a chronic autoimmune disease characterized by inflammation and joint destruction. Recent studies have revealed AOM's efficacy in inhibiting tumor necrosis factor-alpha (TNFα) -mediated inflammation, a crucial pathway involved in RA pathogenesis. AOM's anti-inflammatory effects are attributed to its ability to; inhibit inducible nitric oxide synthase (iNOS) activity, block the iNOS/ERK/p65 signaling pathway, suppress NF-κB activation and p65 translocation, reduce inflammatory cytokine production. According to our research, AOM is therapeutic against CIA via inhibition of the iNOS/ERK/p65 signaling pathway after binding with iNOS.
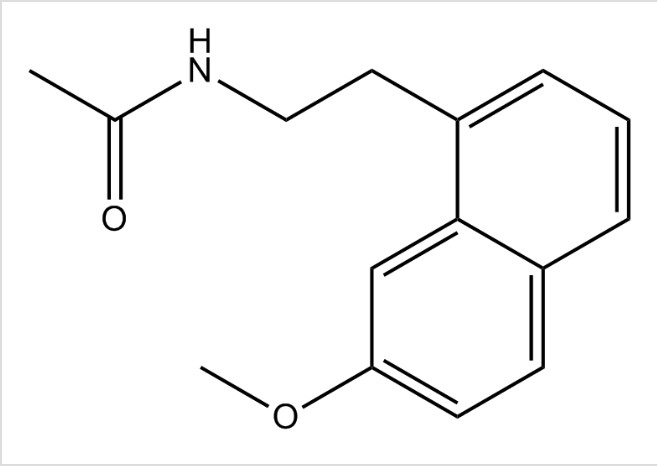
Fig 1. Shows the chemical structure of Agomelatine
Agomelatine's Efficacy in Treating Rheumatoid Arthritis: Agomelatine (AOM) has shown promising results in treating rheumatoid arthritis (RA) in a collagen-induced arthritis (CIA) mouse model. Five drugs, AOM, CCC, ALM, SV, and PDL, were identified as inhibitors of fibroblast. However, AOM demonstrated significant efficacy in alleviating joint swelling, comparable to methotrexate (MTX), a commonly used RA treatment. ALM, CCC, and PDL showed moderate efficacy, while SV had no significant effect. Notably, AOM reduced inflammation and bone erosion in both knee and ankle joints, similar to MTX. The therapeutic effects of AOM were evaluated alongside MTX, which served as a positive control. The results indicate that Agomelatine may be a valuable addition to RA treatment options, offering a novel therapeutic avenue for patients.
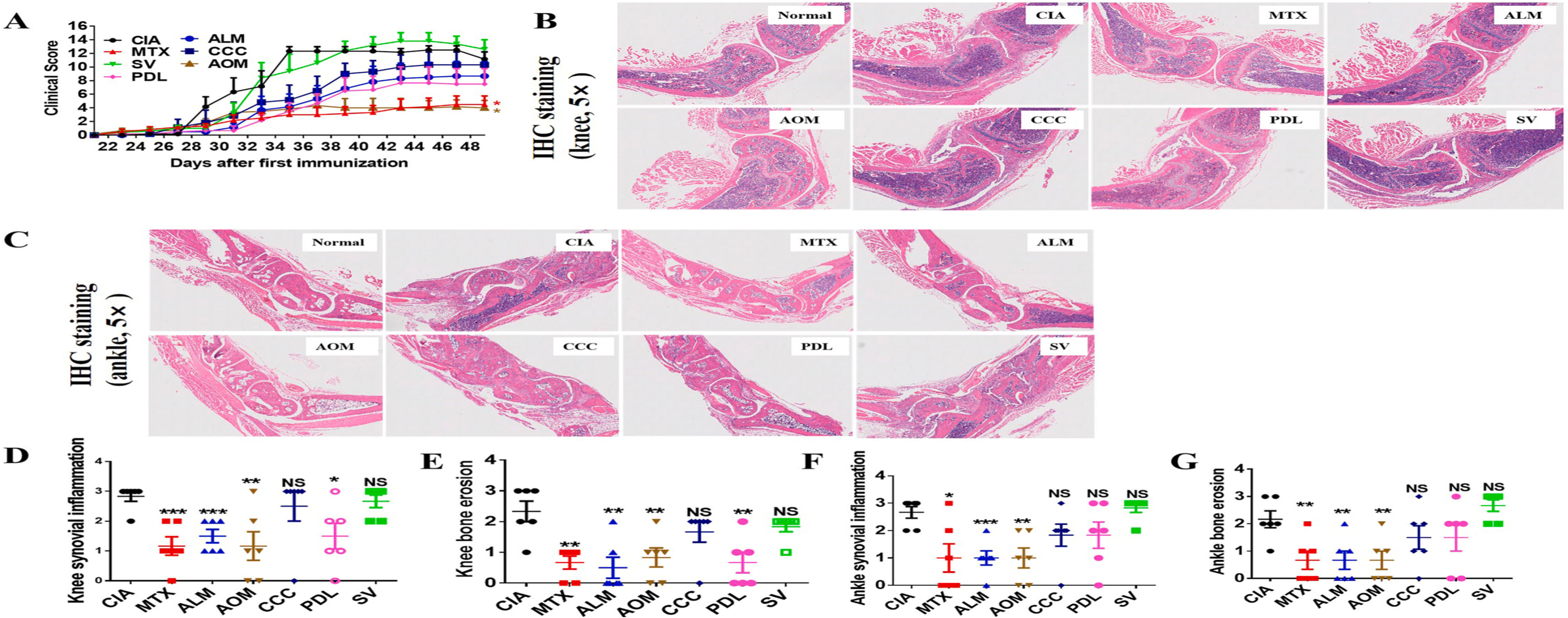
Fig. 2. Therapeutic effects of the five identified drugs on CIA mouse models. CIA mouse models were established to test the treatment effect of SV (10 mg/kg), PDL (10 mg/kg), ALM (5 mg/kg), CCC (10 mg/kg), and AOM (10 mg/kg), with MTX (2 mg/kg) as the positive control. (A) Clinical score for joint swelling, (B) H&E staining of knee joint, (C) H&E staining of ankle joint, and (D–G) pathological scoring of H&E staining for joint inflammation and bone erosion.
Agomelatine's Anti-Inflammatory Mechanism Induced by Cytokine production: Research on Agomelatine (AOM) revealed its potential as a therapeutic agent in treating rheumatoid arthritis, particularly in the collagen-induced arthritis (CIA) mouse model. Notably, Agomelatine significantly reduced joint swelling and inflammation. Further investigation using NF-κB luciferase assays demonstrated that AOM inhibits TNFα-induced NF-κB activity in a dose-dependent manner (Fig. S4B). Considering macrophages' critical role in rheumatoid arthritis pathology, researchers examined AOM's impact on inflammatory cytokine production in RAW264.7 macrophage cells and fibroblast-like synoviocytes (FLS). This research found that AOM decreases mRNA expression and secretion levels of IL-1β, IL-6, and IL-8 in both RAW264.7 cells and FLS . These findings highlight AOM's capacity to suppress key inflammatory mediators, underscoring its therapeutic potential for rheumatoid arthritis.
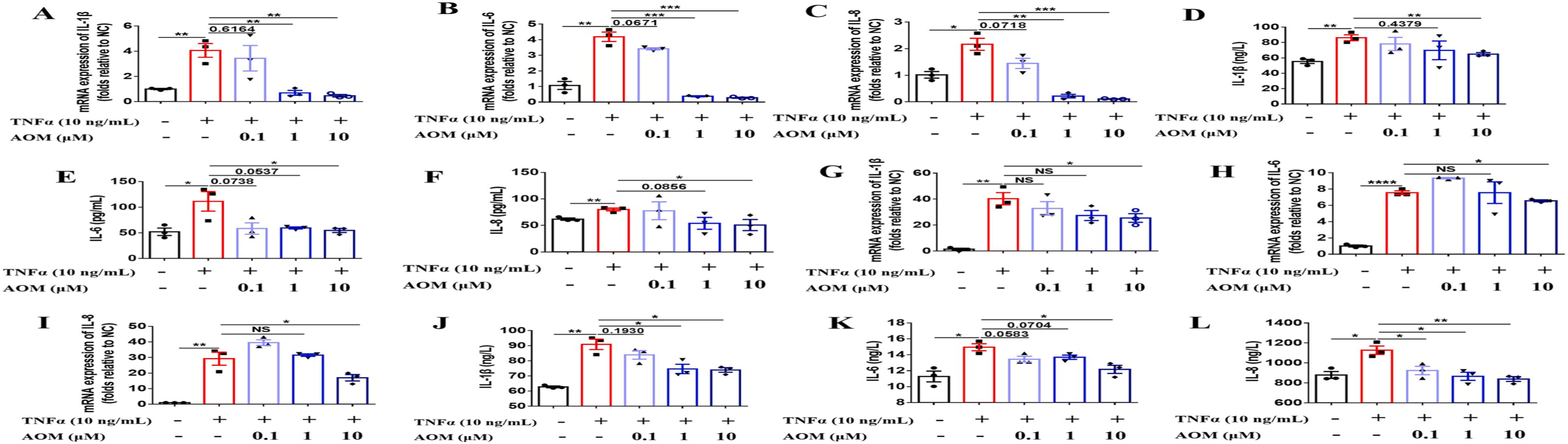
Fig. 3. Agomelatine inhibits TNFα-induced inflammatory cytokine production. Cells were seeded in 6-well plates, then AOM (0.1, 1, and 10 μM) and TNFα (10 ng/mL) were added. Cells were left for 12 or 24 h and collected to test the mRNA expression or secretion levels of inflammatory cytokines IL-1β, IL-6, and IL-8 via qRT-PCR or ELISA. (A–F) Inhibitory effect of AOM on inflammatory cytokine levels in RAW264.7 cells. (G–L) Inhibitory effect of AOM on inflammatory cytokine levels in FLS.
Agomelatine's Impact on FLS Functions, CIA Mouse Models, and iNOS Inhibition: Agomelatine (AOM) showed therapeutic potential in rheumatoid arthritis by modulating fibroblast-like synoviocyte (FLS) functions and inhibiting inducible nitric oxide synthase (iNOS). AOM inhibited FLS proliferation, migration, and TNFα-induced inflammation. In collagen-induced arthritis (CIA) mouse models, Agomelatine (2-10 mg/kg) reduced clinical scores, histological inflammation, and TNFα, IL-1β, IL-6, and IL-8 levels, comparable to methotrexate. Bioinformatics confirmed AOM's binding to iNOS, reducing its expression and activity. The iNOS inhibitor 1400 W (2-10 mg/kg) replicated AOM's therapeutic effects, reducing clinical scores, inflammation, and cytokine levels. Both AOM and 1400 W decreased iNOS levels and activity, underscoring Agomelatine's potential as a rheumatoid arthritis treatment.
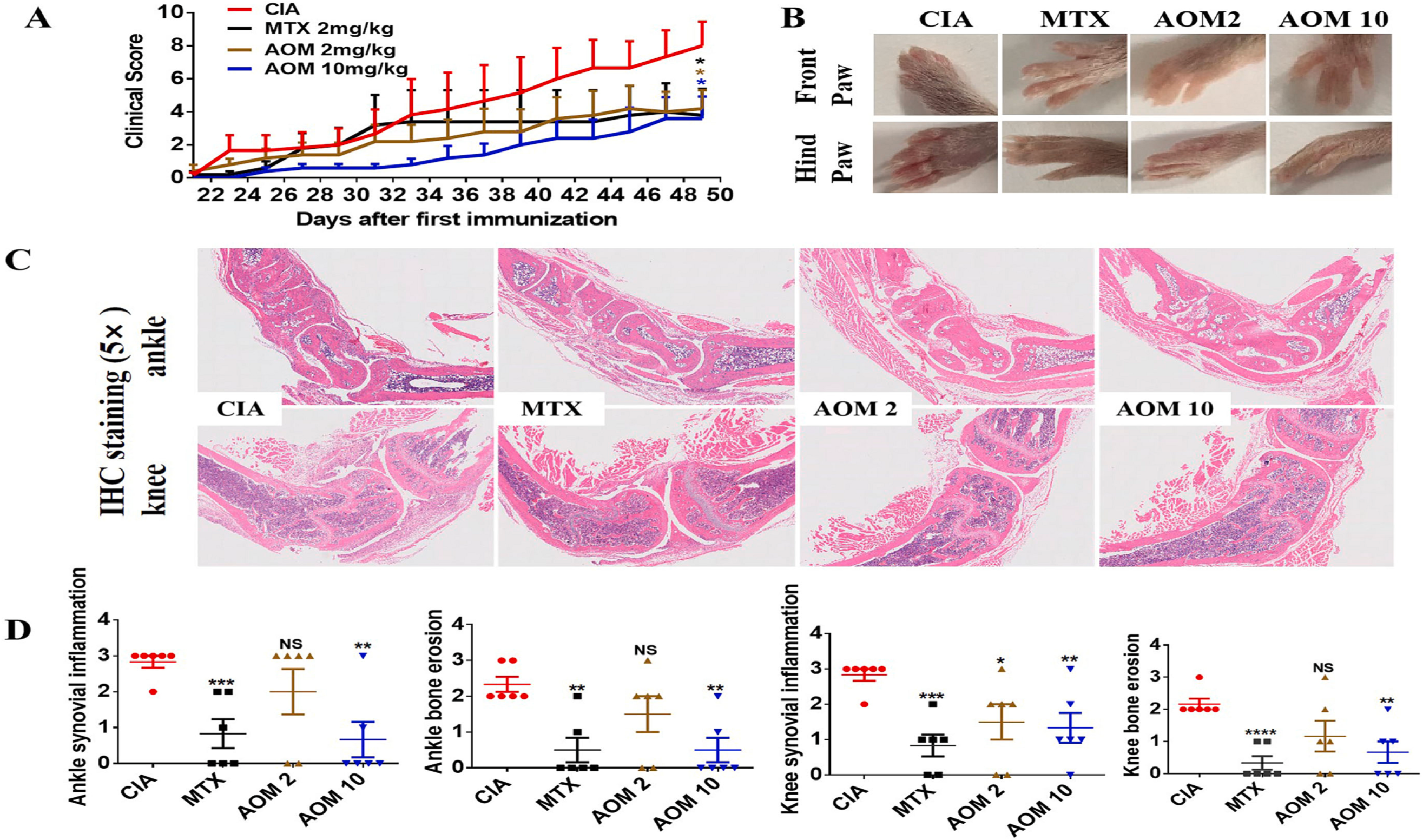
Fig. 4. Therapeutic effects of Agomelatine on CIA mouse models, which were established to test the treatment effect of AOM (at 2 mg/kg or 10 mg/kg), with MTX (2 mg/kg) as a positive control. (A) Clinical score indicating joint swelling. (B) Representative figures demonstrating paw swelling in different groups. (C) H&E staining of knee and ankle joints. (D) Pathological scoring of joint inflammation and bone erosion from H&E staining.
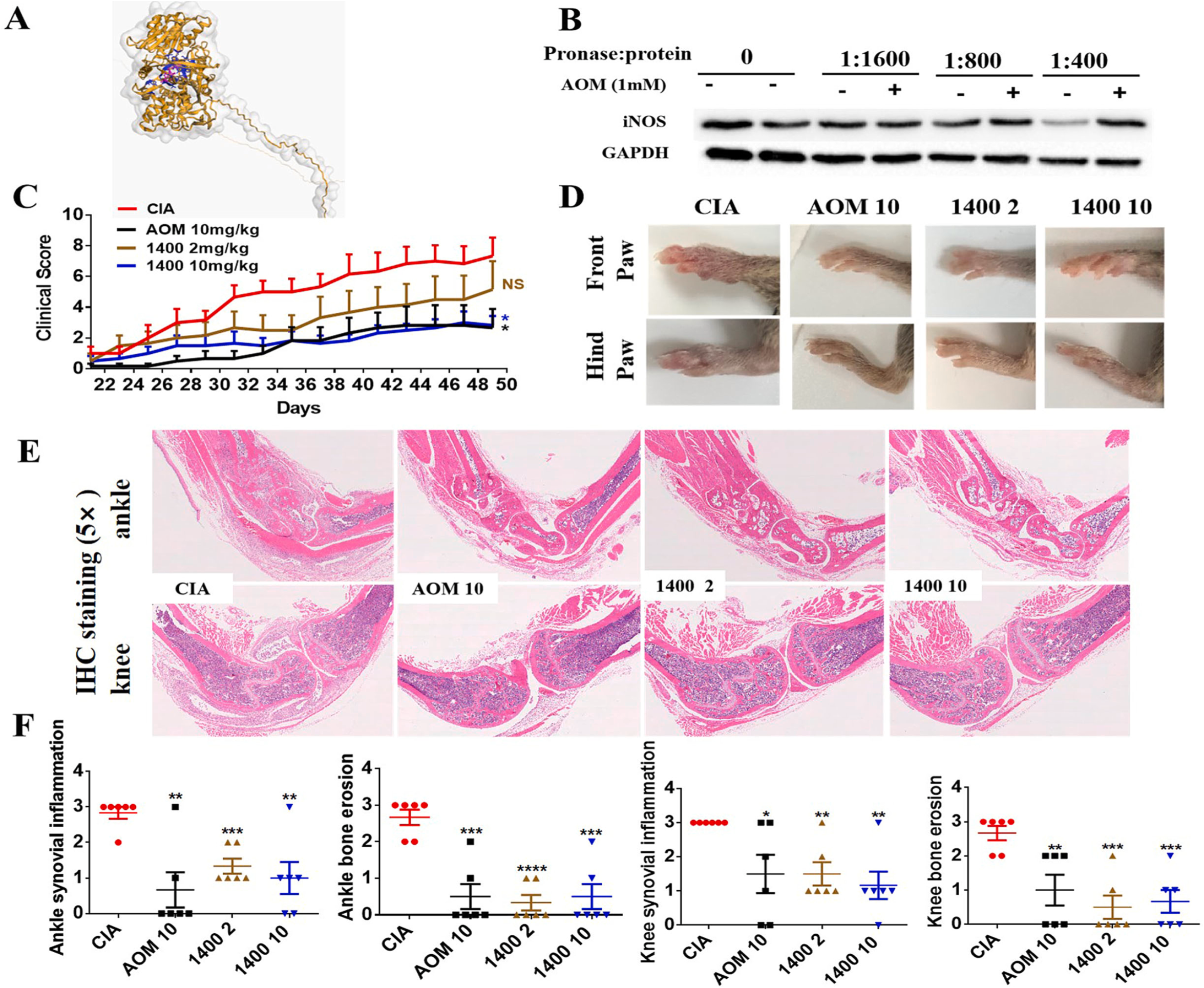
Fig. 5. iNOS inhibitor, 1400 W, is therapeutic in CIA mouse models. The treatment effect of 1400 W (2 mg/kg and 10 mg/kg) was tested with AOM (10 mg/kg) as a positive control. (A) Visualization of the binding between Agomelatine and the predicted target iNOS. Yellow structures and grey surface indicate the iNOS structure, blue structures indicate the amino acids with which AOM binds to iNOS, and pink structures denote AOM structures. (B) DARTs assay was used to test the binding between Agomelatine and iNOS. (C) Clinical score for joint swelling. (D) Representative figures indicating paw swelling in different groups. (E) H&E staining of knee and ankle joints. (F) Pathological scoring indicating joint inflammation and bone erosion using H&E staining.
Agomelatine's Mechanism: Inhibition of iNOS/ERK/p65 Signaling Pathway: Agomelatine (AOM) inhibits TNFα-induced NF-κB activation and inflammatory cytokine production through the iNOS/ERK/p65 signaling pathway. Immunofluorescence staining and western blotting revealed that AOM (10 μM) suppressed TNFα-induced p65 translocation in RAW264.7 cells . Agomelatine reduced ERK phosphorylation, but not JNK or p38, in bone marrow-derived macrophages (BMDMs) . Immunohistochemistry (IHC) staining showed increased phosphorylation of ERK and p65, and iNOS levels in CIA model ankle slides, which AOM decreased by reducing synoviocyte proliferation . To confirm Agomepatine's mechanism, iNOS inhibitor 1400 W and NF-κB inhibitor JSH-23 were used with/without AOM in RAW264.7 cells and FLS. Results indicated that 1400 W (10 μM), JSH-23 (10 μM), and Agomelatine (10 μM) decreased IL-1β, IL-6, and IL-8 mRNA expression and secretion levels alone. However, Agomelatine did not further reduce cytokine production in the presence of 1400 W or JSH-23 . These findings suggest that Agomelatine's inhibitory effect on inflammatory cytokine production is partially dependent on the iNOS/ERK/p65 signaling pathway.
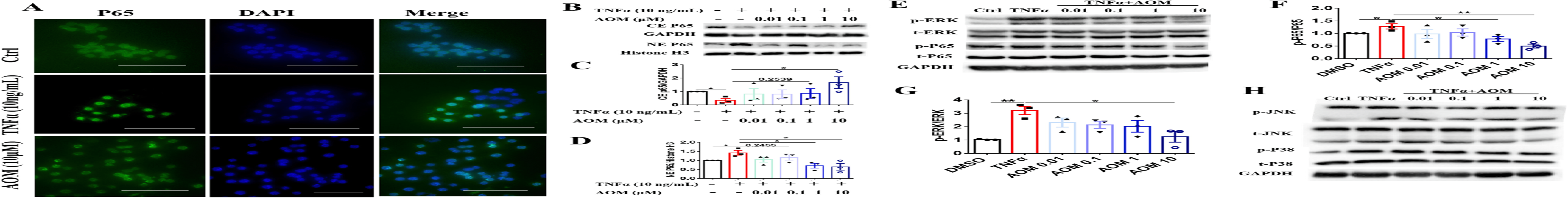
Fig. 6. Shows the events how Agomelatine inhibits the iNOS/ERK/p65 signaling pathway.
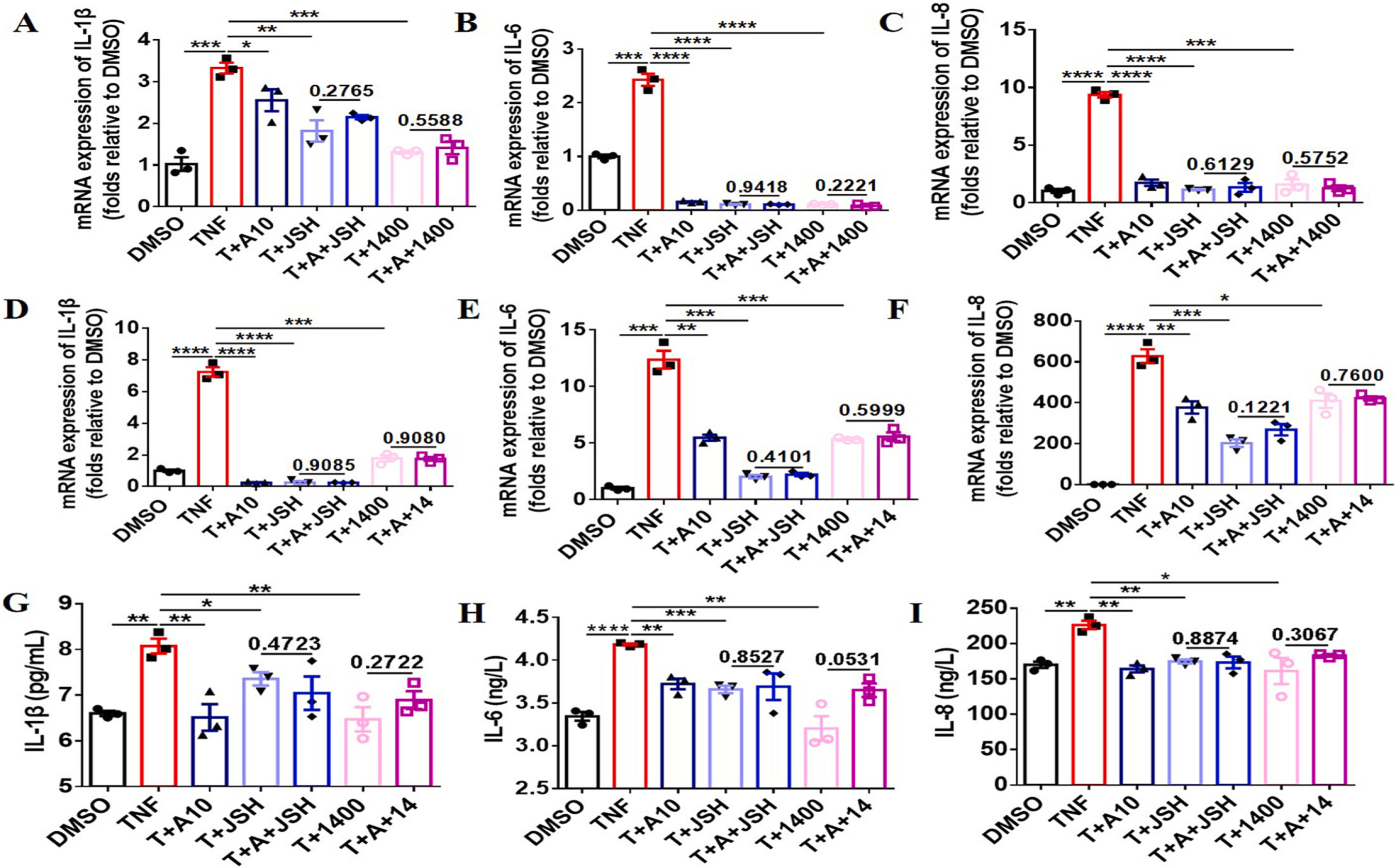
Fig. 7. Inhibitory effect of AOM on inflammatory cytokine production is dependent on the iNOS/ERK/p65 signaling pathway. Cells were seeded in 6-well plate and AOM (10 μM), JSH-23 (10 μM), 1400 W (10 μM), and TNFα (10 ng/mL) were added for 12 or 24 h. Cells were then collected and qRT-PCR or ELISA was performed to test mRNA expression or the secretion levels of inflammatory cytokines, IL-1β, IL-6, and IL-8. (A–C) mRNA expression levels of inflammatory cytokines in RAW264.7 cells following TNFα stimulation for 12 h. (D–F) mRNA expression levels in FLS inflammatory cytokines following TNFα stimulation for 12 h. (G–I) Secretion levels of FLS inflammatory cytokines following TNFα stimulation for 24 h. Three independent experiments were performed.
In short Agomelatine is traditionally used to treat depression by targeting serotonin receptors. Our research reports that AOM can also be used to treat diseases that are predominantly the result of TNFα-stimulated NF-κB activation, such as arthritis, through in hibition of iNOS/ERK/p65 signaling pathway by targeting iNOS.















Commentaires