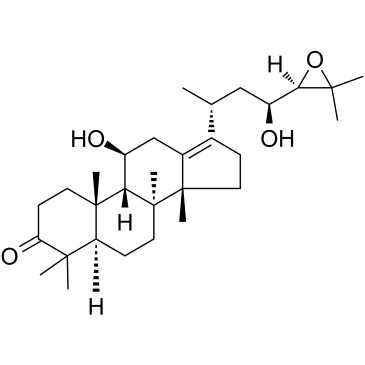
Alisol B
Alisol B (ALB), a triterpenoid compound extracted from Alisma, has been reported to ameliorate nonalcoholic steatohepatitis (NASH) and slow obesity. However, the effect of ALB on hyperlipidemia and mechanism are unclear. Alisol B (ALB) a triterpenoid compound identified as one of the earliest triterpenoids constituents in Alisma orientalis . Alisol B has been found to exhibit a range of biological activities , including regulating RARα-PPARγ-CD36 cascade to inhibit fatty acid uptake and improve non-alcoholic steatohepatitis (NASH) and activating AMPK to inhibit PPARγ and C/EBPα and improve HFD-induced obesity by inhibiting adipocyte differentiation and maturation .

Figure 1 : Shows the chemical structure of Alisol B.
Alisol B ameliorates hyperlipidemia and hepatic steatosis in vivo and in vitro :Alisol B treatment significantly reduced the accumulation of intracellular lipid droplet in OA-induced hepatocytes, as demonstrated by staining with nile red and BODIPY 493/503 . Consistently, Alisol B treatment dose-dependently decreased the intracellular TC, TG level, with TC levels returning to normal after treatment with 15 µM Alisol B . Additionally, Alisol B increased HDL-c and decreased LDL-c in blood. Liver injury and steatosis, indicated by the levels of AST and ALT were effectively improved by various doses of Alisol B . Alisol B also demonstrated a reduction in hepatic lipid deposition and hepatic steatosis . Collectively, these findings suggest that ALB improves hyperlipidemia and hepatic steatosis.

Fig. 2. Shows the efficacy of Alisol B in improving lipid metabolism disorders both in vivo and in vitro.
ALB inhibit SREBPs to improve hyperlipidemia : To explore potential mechanism of Alisol B on hyperlipidemia, the researchers performed the RNA-seq analysis in WD-induced mice. The researchers examined the expression of SREBPs in the liver of WD mice. Treatment with different doses of Alisol B effectually reduced the expression of n-SREBP-1 and n-SREBP-2 in the liver of WD-mice . Furthermore, Alisol B treatment gradually decreased the expression of fatty acid synthesizing genes, such as fasn (p<0.01), and cholesterol synthesizing genes, such as hmgcr (p<0.05), ect, in a dose-dependent manner (Fig. 3G-H). It is worth noting that these genes are the target genes of SREBPs. These results indicate that ALB inhibits SREBPs to improve hyperlipidemia.

Fig. 3. ALB inhibits SREBPs to improve lipid metabolism disorders. (A-D) GSEA pathways analysis in the liver of WD- mice (n = 3). (E) Western blot of SREBP-1 and SREBP-2 in the liver of WD-mice. (F) Relative protein expression of the n-SREBP-1 and n-SREBP-2 in (E). (G and H) Relative mRNA expression of SREBPs and its target genes in the liver of WD-mice. ALBH (WD with 50 mg/kg/d ALB).
Impact of ALB on SREBPs in vitro setting: Alisol B inhibited the luciferase activity of SREBPs in a dose- and time-dependent manner. Notably, 15 μM Alisol B showed an inhibition rate of more than 50 % after 9 h administration (Fig. 4 ). In order to eliminate the inhibitory effect of ALB on SREBPs due to cell damage, researchers used MTT to detect the damage of ALB to hepatocytes. Shown in Fig. 4C-D, Alisol B does not cause any damage to the cell when it is less than 15 μM. Furthermore, ALB decreased the expression of n-SREBP-1 and n-SREBP-2 proteins in HepG2 and HL7702 cells in a dose- and time-dependent manner (Fig. 4E-J). Consistent with this, immunofluorescence analysis revealed that ALB decreased the localization of SREBP-1 or SREBP-2 with the nucleus (Fig. 4K and L). Consistent with the above findings in WD-mice, ALB consistently suppressed the target genes associated with SREBPs, namely HMGCR,PCSK9, MVK, FASN, and ACC1 which are closely associated with the de novo synthesis of cholesterol and fatty acid (Fig. 4M-N). Cholesterol plays a vital role in cellular growth and survival. In the absence of cholesterol in the medium, cell growth and survival rely on intracellular cholesterol synthesis. As demonstrated in Fig. 4O, the absence of cholesterol resulted in a dose-dependent inhibition of cell growth by ALB. However, this inhibitory effect was counteracted upon the introduction of exogenous cholesterol, indicating that ALB impeded the synthesis of endogenous cholesterol. Therefore, these findings provide evidence that ALB effectively inhibits lipid synthesis and SREBPs both in vitro and in vivo.

Fig. 4 . ALB inhibits SREBPs transcription, reduces SREBPs mRNA and protein expressionALB blocks SREBPS/SCAP transport from ER to GA
ALB blocks SREBPS/SCAP transport from ER to GA : To investigate the mechanism through which ALB reduces the levels of n-SREBPs, we explored if Alisol B influences degradation of n-SREBPs by overexpression of n-SREBPs. The results showed that ALB significantly reduced the level of endogenous n-SREBP-1 and n-SREBP-2, but had no effect on exogenous Flag-n-SREBP-1 and Flag-n-SREBP-2 (Fig. 5A-5D). Additionally, Alisol B did not accelerate the degradation of n-SREBP-1 and n-SREBP-2 when treated with the protein synthesis inhibitor cycloheximide (CHX, Fig. 5E-H), implicating that ALB functions prior to the maturation of SREBPs. Researchers investigated the potential impact of ALB on the activation of the cleavage process of SREBPs by examining the alteration in phosphatase substrate content in medium of CHO-K1 cells, a cell with the secretory function. These cells were genetically modified to express a fusion protein consisting of secreted alkaline phosphatase (ALP) and a truncated SREBP-2 fragment (PLAP-BP2513–1141) lacking the NH2 terminal DNA-binding domain, which allowed us to monitor the translocation of ER-GA (Fig.4I). Co-transfection of plasmids encoding PLAP-BP2513–1141 and SCAP in the cells resulted in the secretion of PLAP phosphatase upon serum lipoprotein deficiency, and the presence of ALB in the medium led to a reduction in ALP content, consistent with 25-HC, an SREBPs inhibitor that directly binds to block the pre-SREBPs/SCAP complex separate from the ER (Fig. 5J). Conversely, ALB does not appear to have any impact on the expression of SCAP and Insigs, both of which are known to play a crucial role in the cleavage activation process of SREBPs (Fig. 5K). Given that SCAP is responsible for the transportation of SREBPs from the ER to the GA, we proceeded to use immunofluorescence to investigate the influence of ALB on the transport of the pre-SREBPs-SCAP complex. The co-localization of SCAP with GA-marker protein GM130 was observed to gradually decrease, while its co-localization with the ER-marker protein Calnexin was observed to increase, as depicted in Fig. 5N–O. These findings suggest that Alisol B inhibits the transportation of SREBPs from ER to the GA.
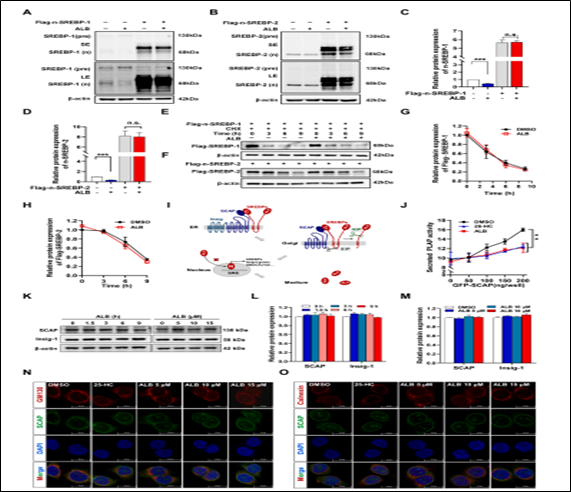
Fig. 5 . ALB reduces cleavage activation of SREBPs though inhibiting the transport of the SCAP/SREBPs complex from ER to GA.
Thus the researchers have successfully identified ALB as a small molecule inhibitor of SREBPs. ALB demonstrated efficacy in improving the classic hyperlipidemia model induced by WD and in reducing lipid accumulation induced by OA. These findings provide valuable insights for the development of novel lipid-lowering drugs. Furthermore, based the results obtained in our study, we propose that ALB exerts its regulatory effects on the AMPK/mTORs/SREBPs signaling pathway by specifically targeting VDAC1, thereby alleviating hyperlipidemia. (Fig 6.)

Fig. 6. Mechanism of ALB improving hyperlipidemia. ALB targets VDAC1 and establishes two hydrogen bonds between the amino acid sites S196 and H184, which also are crucial site for ATP transport in VDAC1. This interaction impedes the transfer of ATP from mitochondria to cytoplasm, leading to an elevation in the ADP/ATP and AMP/ATP ratios. Consequently, the activation of AMPK is triggered, thereby regulating the mTOR/p70S6K pathway. Subsequently, the inhibition of SREBPs cleavage activation from the endoplasmic reticulum to the Golgi apparatus occurs, ultimately resulting in the suppression of lipid synthesis gene expression and exerting a lipid-lowering effect.















Commentaires