Olmutinib (HM61713, BI 1482694) (Synonyms: BI-1482694, Olmutinib) |
| Catalog No.GC15370 |
L'olmutinib (HM61713, BI 1482694) (HM61713; BI-1482694) est un troisième inhibiteur de la tyrosine kinase EGFR actif et irréversible qui se lie À un résidu cystéine près du domaine kinase. L'olmutinib (HM61713, BI 1482694) est utilisé pour le NSCLC.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 1353550-13-6
Sample solution is provided at 25 µL, 10mM.
GI50: 9.2 and 10 nM for H1975 (L858-T790M) and HCC827 (exon 19 del.), respectively.
Olmutinib (HM61713, BI 1482694) is an EGFR mutant-specific inhibitor.
The tyrosine kinase inhibitors (TKI) against epidermal growth factor receptor (EGFR) are widely used in patients with non-small cell lung cancer (NSCLC). However, EGFR T790M mutation leads to resistance to most clinically available EGFR TKIs. Third-generation EGFR TKIs against the T790M mutation have been in active clinical development.
In vitro: Olmutinib has been identified as an irreversible kinase inhibitor and could covalently bind to a cysteine residue near the kinase domain of mutant EGFR. Olmutinib had a half-life of over 24h for EGFR inhibition. Olmutinib was able to cause potent inhibition in cell lines H1975 (L858R and T790M) and HCC827 (exon 19 deletion). Olmutinib showed a low potency for NSCLC cell line H358 with wild-type EGFR [1].
In vivo: The previous in vivo studies of xenograft models with grafts of H1975 and HCC827 showed that olmutinib was active against the tumors without dasplaying any side effects [1].
Clinical trial: In the ongoing phase I/II study of olmutinib in patients with advanced NSCLC who had failed previous EGFR TKIs, EGFR mutated patients received olmutinib doses ranging from 75 to 1200 mg/day. In the phase II expansion part of the study, 800 mg QD was the dose given to patients. The ORR was 58.8% in the 34 patients who received olmutinib with a dose more than 650 mg. Moreover, 10 patients had unconfirmed partial responses, and 13 showed disease stabilization. DLTs included GI symptoms and elevation of alanine aminotransferase, aspartate aminotransferase, amylase, and lipase. Thus, olmutinib could represent another promising drug for patients with T790M-positive NSCLC [1].
Reference:
[1] Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol. 2016 Apr 12;9:34.
| Cas No. | 1353550-13-6 | SDF | |
| Synonymes | BI-1482694, Olmutinib | ||
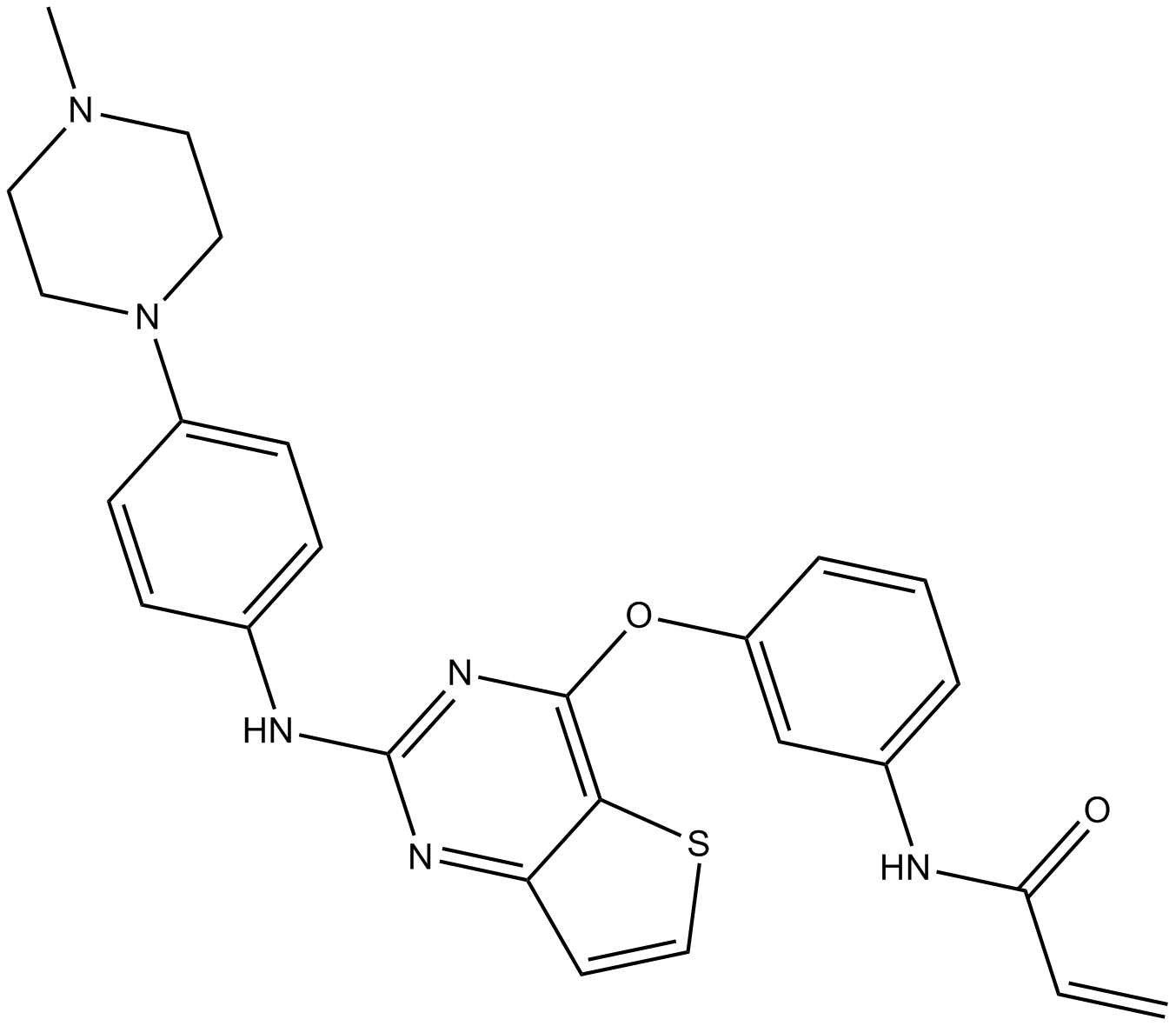
| Chemical Name | N-(3-((2-((4-(4-methylpiperazin-1-yl)phenyl)amino)thieno[3,2-d]pyrimidin-4-yl)oxy)phenyl)acrylamide | ||
| Canonical SMILES | C=CC(NC1=CC=CC(OC2=C3C(C=CS3)=NC(NC4=CC=C(N5CCN(C)CC5)C=C4)=N2)=C1)=O | ||
| Formula | C26H26N6O2S | M.Wt | 486.59 |
| Solubility | DMSO: 44 mg/mL | Storage | Store at -20°C |
| General tips | Please select the appropriate solvent to prepare the stock solution according to the
solubility of the product in different solvents; once the solution is prepared, please store it in
separate packages to avoid product failure caused by repeated freezing and thawing.Storage method
and period of the stock solution: When stored at -80°C, please use it within 6 months; when stored
at -20°C, please use it within 1 month. To increase solubility, heat the tube to 37°C and then oscillate in an ultrasonic bath for some time. |
||
| Shipping Condition | Evaluation sample solution: shipped with blue ice. All other sizes available: with RT, or with Blue Ice upon request. | ||
| Prepare stock solution | |||

|
1 mg | 5 mg | 10 mg |
| 1 mM | 2.0551 mL | 10.2756 mL | 20.5512 mL |
| 5 mM | 0.411 mL | 2.0551 mL | 4.1102 mL |
| 10 mM | 0.2055 mL | 1.0276 mL | 2.0551 mL |
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
 g
g
 μL
μL

Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
3. All of the above co-solvents are available for purchase on the GlpBio website.
Quality Control & SDS
- View current batch:
- Purity: >98.00%
- COA (Certificate Of Analysis)
- SDS (Safety Data Sheet)
- Datasheet
Average Rating: 5 (Based on Reviews and 38 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *



















