Sinefungin (trifluoroacetate salt) (Synonyms: A 9145,Adenosyl-ornithine,Antibiotic A 9145) |
| Catalog No.GC91760 |
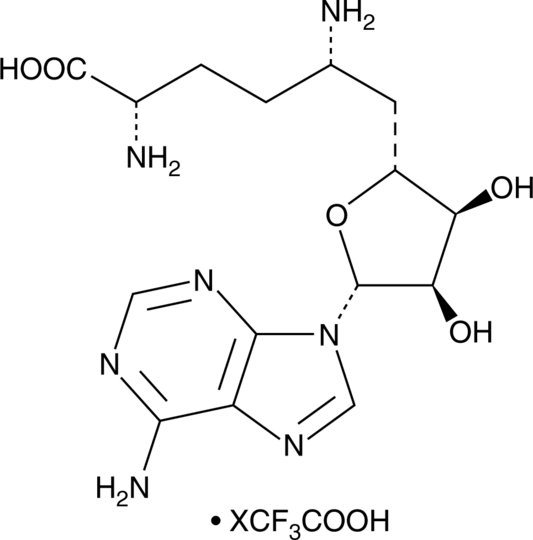
Sinefungin is a purine nucleoside and derivative of S-adenosylhomocysteine that has been found in Streptomyces and has diverse biological activities.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: N/A
Sample solution is provided at 25 µL, 10mM.
Sinefungin is a purine nucleoside and derivative of S-adenosylhomocysteine that has been found in Streptomyces and has diverse biological activities.[1],[2] It inhibits G9a methyltransferase and SET domain-containing protein 7 (SET7) with IC50 values of 10.4 and 2.38 µM, respectively, using histone H3 (1-21) peptide as a substrate and 150 and 9.1 µM, respectively, using full-length histone H3 as a substrate.[3] It also inhibits protein arginine methyltransferase 5 (PRMT5) with IC50 values of 0.31 and 0.69 µM using histone H4 (1-21) peptide and full-length histone H4, respectively, as substrates. Sinefungin (10 mg/kg) reduces renal levels of the mesenchymal marker α-smooth muscle actin (α-SMA), fibrosis markers ferroptosis suppressor protein 1 (Fsp1), collagen 1, collagen 3, and fibronectin, and monomethylation of histone H3 lysine 4 (H3K4me1) in a mouse model of unilateral ureteral obstruction.[4]
References:
[1].Nolan, L.L.Molecular target of the antileishmanial action of sinefunginAntimicrob. Agents Chemother.31(10)1542-1548(1987).
[2].Maguire, M.P., Feldman, P.L., and Rapoport, H.Stereoselective synthesis and absolute stereochemistry of sinefunginJ. Org. Chem.55(3)948-955(1990).
[3].Horiuchi, K.Y., Eason, M.M., Ferry, J.J., et al.Assay development for histone methyltransferasesAssay Drug Dev. Technol.11(4)227-236(2013).
[4].Sasaki, K., Doi, S., Nakashima, A., et al.Inhibition of SET domain-containing lysine methyltransferase 7/9 ameliorates renal fibrosisJ. AM. Soc. Nephrol.27(1)203-215(2016).
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















