ALW-II-41-27 (Synonyms: Eph receptor tyrosine kinase inhibitor;) |
| カタログ番号GC11134 |
ALW-II-41-27はEphファミリーチロシンキナーゼ阻害剤であり、IC50値11nMでEph2を阻害する
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 1186206-79-0
Sample solution is provided at 25 µL, 10mM.
ALW-II-41-27はEphファミリーチロシンキナーゼ阻害剤であり、IC50値11nMでEph2を阻害する。
ALW-II-41-27(1μM;72時間)はエルロチニブ耐性NSCLC細胞株の増殖を阻害し、細胞アポトーシスを増加させた。ALW-II-41-27はアポトーシスを誘導したが、カスパーゼ-3とPARPの増加、抗アポトーシス蛋白質BCL-xLとMCL-1の発現の減少が伴った。ALW-II-41-27(200, 600, 1,000nMのALW-II-41-27; 24, 48, 72時間)はRhoA/ROCKの伝達経路を阻害することで子宮頸がん(CC)細胞の増殖、遊走、浸潤を阻害した。ALW-II-41-27はpY772-EphA2を阻害したが、EphA2-Y772AはNPC細胞増殖におけるALW-II-41-27の阻害効果を減少させた。ALW-II-41-27 とcetuximabの併用治療はcetuximabに対する一次抵抗および獲得耐性を回復させ、また細胞増殖を阻害し、アポトーシスと細胞周期G1-G2の停止を引き起こした。
ALW-II-41-27(15 mg/kg;14日間; i.p.)はエルロチニブ耐性腫瘍の成長を著しく抑制した。ALW-II-41-27(15, 30 mg/kg;1日2回; i.p.)の投与は腫瘍を持つマウスにおいてH358腫瘍の増殖を著しく抑制した。病理組織学的検査により、NG-25または担体物質と比べALW-II-41-27の投与した腫瘍はアポトーシスを有意に増加し、EPHA2の遺伝子破壊の効果と同様であった。ALW-II-41-27 (12.5, 25, 50, 100 μg/kg; i.p.)は消化管運動と腹部撤退反射(AWR)のスコアを低減させ、酸化ストレスマーカー[4-ヒドロキシ-2-ノネナール(4-HNE)、プロテインカルボニル、8-ヒドロキシ-2-デアキシグアニン(8-OHdG)]および炎症性サイトカイン(TNF-α、IL-6、IL-17、ICAM-1)のレベルを有意に低下させ、三日寄生虫感染マウスの大腸と血清において抗炎症サイトカイン(IL-10)のレベルを上昇させた。
References:
[1]. Choi Y, Syeda F, et,al. Discovery and structural analysis of Eph receptor tyrosine kinase inhibitors. Bioorg Med Chem Lett. 2009 Aug 1;19(15):4467-70. doi: 10.1016/j.bmcl.2009.05.029. Epub 2009 May 13. PMID: 19553108; PMCID: PMC2730633.
[2]. Amato KR, Wang S, et,al. Genetic and pharmacologic inhibition of EPHA2 promotes apoptosis in NSCLC. J Clin Invest. 2014 May;124(5):2037-49. doi: 10.1172/JCI72522. Epub 2014 Apr 8. PMID: 24713656; PMCID: PMC4001547.
[3]. Amato KR, Wang S, et,al. EPHA2 Blockade Overcomes Acquired Resistance to EGFR Kinase Inhibitors in Lung Cancer. Cancer Res. 2016 Jan 15;76(2):305-18. doi: 10.1158/0008-5472.CAN-15-0717. Epub 2016 Jan 7. PMID: 26744526; PMCID: PMC4715957.
[4]. Li X, Li D, et,al.ALW-II-41-27, an EphA2 inhibitor, inhibits proliferation, migration and invasion of cervical cancer cells via inhibition of the RhoA/ROCK pathway. Oncol Lett. 2022 Apr;23(4):129. doi: 10.3892/ol.2022.13249. Epub 2022 Feb 18. PMID: 35251349; PMCID: PMC8895465.
[5]. Zeng L, Li K, et,al.A Novel EphA2 Inhibitor Exerts Beneficial Effects in PI-IBS in Vivo and in Vitro Models via Nrf2 and NF-κB Signaling Pathways. Front Pharmacol. 2018 Mar 27;9:272. doi: 10.3389/fphar.2018.00272. PMID: 29662452; PMCID: PMC5890185.
[6]. Xiang YP, Xiao T, et,al. Y772 phosphorylation of EphA2 is responsible for EphA2-dependent NPC nasopharyngeal carcinoma growth by Shp2/Erk-1/2 signaling pathway. Cell Death Dis. 2020 Aug 27;11(8):709. doi: 10.1038/s41419-020-02831-0. PMID: 32848131; PMCID: PMC7449971.
[7]. Martini G, Cardone C, et,al. EPHA2 Is a Predictive Biomarker of Resistance and a Potential Therapeutic Target for Improving Antiepidermal Growth Factor Receptor Therapy in Colorectal Cancer. Mol Cancer Ther. 2019 Apr;18(4):845-855. doi: 10.1158/1535-7163.MCT-18-0539. Epub 2019 Mar 1. PMID: 30824612.
| 細胞実験[1]: | |
細胞株 | 非小細胞肺がん(NSCLC) PC-9/ER, PC-9/ERC15, PC-9/ERC16細胞株 |
準備方法 | erlotinibに対する耐性をもつ4つの細胞株にALW-II-41-27、NG-25、erlotinib、DMSOで72時間処理し、細胞生存率をMTTアッセイで評価した。 |
反応条件 | 1 µM;72 h |
アプリケーション | 1µMのALW-II-41-27はErlotinib耐性獲得NSCLC細胞株の増殖を阻害し、細胞アポトーシスを増加させた。ALW-II-41-27はアポトーシスを誘導したが、カスパーゼ-3とPARPの増加、抗アポトーシス蛋白質BCL-xLとMCL-1の発現の減少が伴った。 |
| 動物実験 [2]: | |
動物モデル | 6週齢の無胸腺ヌードマウス |
準備方法 | HCC827/ERまたはPC-9/ERC16をマトリゲルとともに6週齢の無胸腺ヌードマウスの後脇腹に注射した。マウスは体重と腫瘍の体積によって無作為に治療群に分け、erlotinib、ALW-II-41-27、またはビークルを15mg/kg、1日2回腹腔内注射した。 |
投与形態 | 15 mg/kg;14日; i.p. |
アプリケーション | 治療14日後、ALW-II-41-27はerlotinib耐性腫瘍の増殖を有意に抑制した |
References:
[1]. Amato KR, Wang S, et,al. EPHA2 Blockade Overcomes Acquired Resistance to EGFR Kinase Inhibitors in Lung Cancer. Cancer Res. 2016 Jan 15;76(2):305-18. doi: 10.1158/0008-5472.CAN-15-0717. Epub 2016 Jan 7. PMID: 26744526; PMCID: PMC4715957.
| Cas No. | 1186206-79-0 | SDF | |
| 同義語 | Eph receptor tyrosine kinase inhibitor; | ||
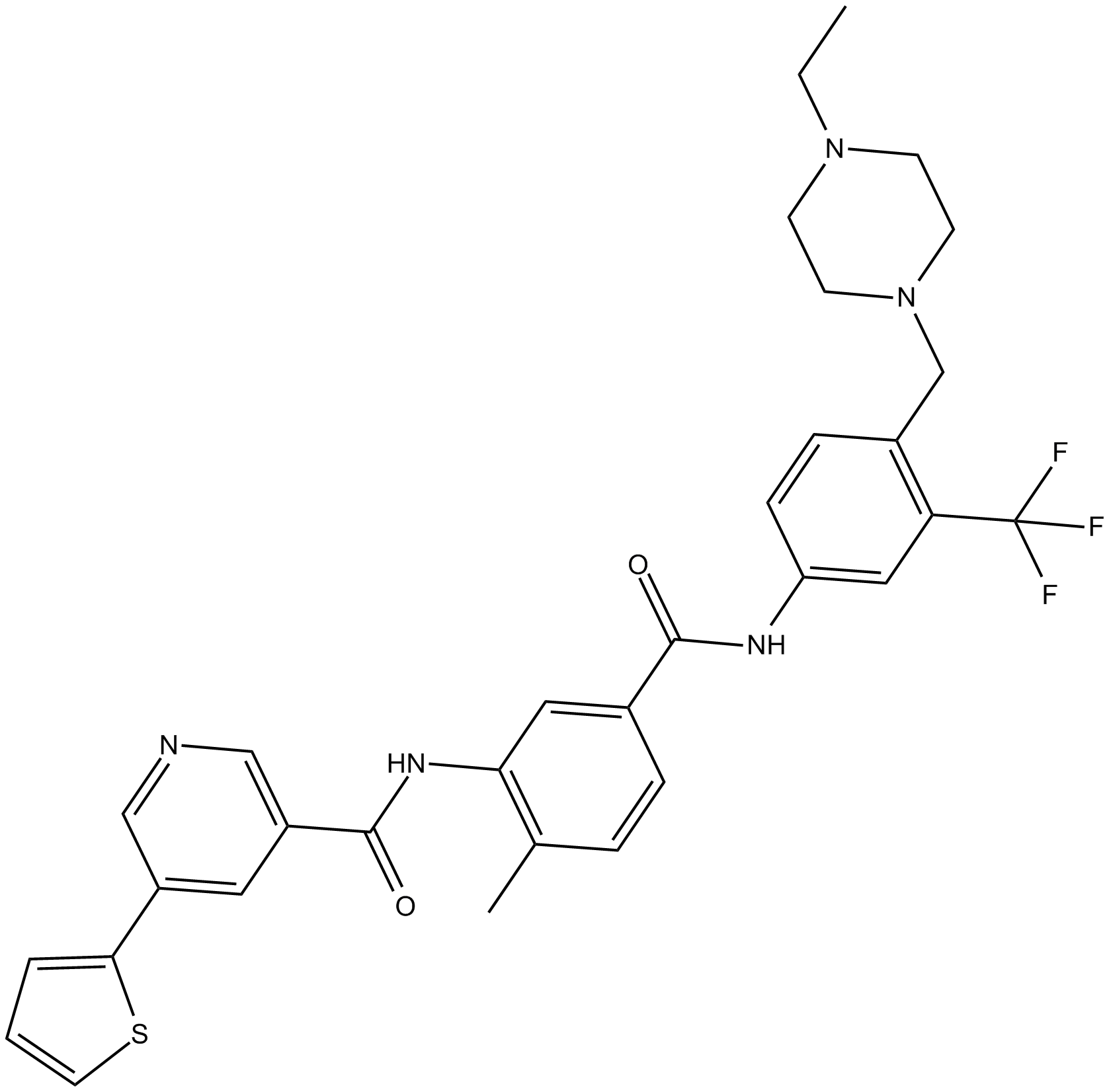
| Chemical Name | N-(5-((4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)carbamoyl)-2-methylphenyl)-5-(thiophen-2-yl)nicotinamide | ||
| Canonical SMILES | CCN1CCN(CC2=C(C(F)(F)F)C=C(NC(C3=CC(NC(C4=CN=CC(C5=CC=CS5)=C4)=O)=C(C=C3)C)=O)C=C2)CC1 | ||
| Formula | C32H32F3N5O2S | M.Wt | 607.69 |
| 溶解度 | ≥ 102 mg/mL in DMSO, ≥ 60.8 mg/mL in EtOH | Storage | Store at -20°C |
| General tips | Please select the appropriate solvent to prepare the stock solution according to the
solubility of the product in different solvents; once the solution is prepared, please store it in
separate packages to avoid product failure caused by repeated freezing and thawing.Storage method
and period of the stock solution: When stored at -80°C, please use it within 6 months; when stored
at -20°C, please use it within 1 month. To increase solubility, heat the tube to 37°C and then oscillate in an ultrasonic bath for some time. |
||
| Shipping Condition | Evaluation sample solution: shipped with blue ice. All other sizes available: with RT, or with Blue Ice upon request. | ||
| Prepare stock solution | |||

|
1 mg | 5 mg | 10 mg |
| 1 mM | 1.6456 mL | 8.2279 mL | 16.4558 mL |
| 5 mM | 0.3291 mL | 1.6456 mL | 3.2912 mL |
| 10 mM | 0.1646 mL | 0.8228 mL | 1.6456 mL |
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
 g
g
 μL
μL

Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
3. All of the above co-solvents are available for purchase on the GlpBio website.
Quality Control & SDS
- View current batch:
- Purity: >98.00%
- COA (Certificate Of Analysis)
- SDS (Safety Data Sheet)
- Datasheet
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *



















