Atrasentan hydrochloride |
| カタログ番号GC11783 |
Products are for research use only. Not for human use. We do not sell to patients.
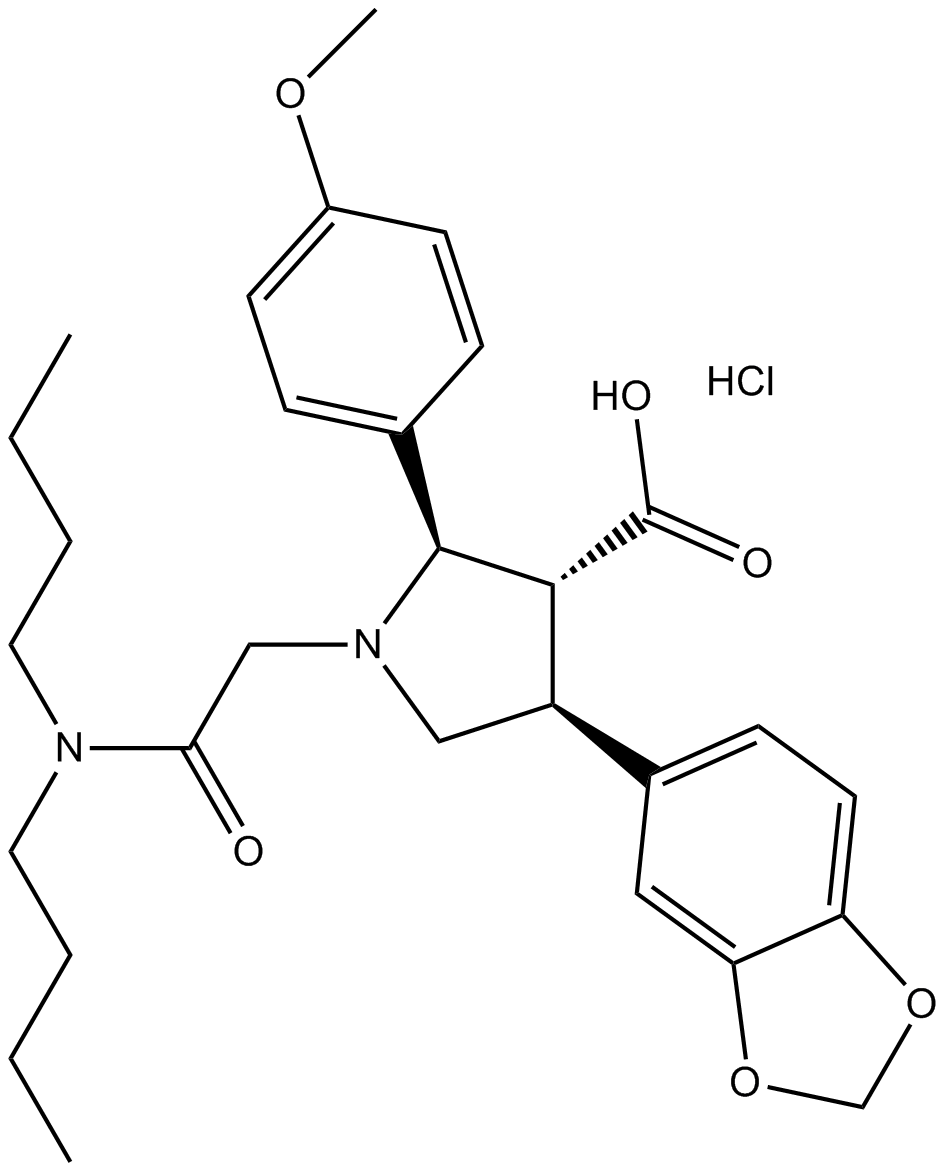
Cas No.: 195733-43-8
Sample solution is provided at 25 µL, 10mM.
Kinase experiment: | Cells are incubated and treated with Atrasentan. They are then washed twice with PBS and lysed in ice-cold lysis buffer [20 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium PPi, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 μg/mL leupeptin, and 1 mM PMSF]. The extracts are centrifuged to remove cellular debris, and the protein content of the supernatants is determined using the bicinchoninic acid (BCA) protein assay reagent. Proteins (150 μg) are incubated with gentle rocking at 4°C overnight with immobilized Akt antibody cross-linked to agarose hydrazide beads. After the Akt is selectively immunoprecipitated from the cell lysates, the immunoprecipitated products are washed twice with lysis buffer and twice with kinase assay buffer [25 mM Tris (pH 7.5), 10 mM MgCl2, 5 mM β-glycerol phosphate, 0.1 mM sodium orthovanadate, 2 mM DTT] and then resuspended in 40 μL of kinase assay buffer containing 200 μM ATP and 1 μg GSK-3α/β fusion protein. The kinase assay reaction is allowed to proceed at 30°C for 30 min and stopped by the addition of Lamelli SDS sample buffer. Reaction products are resolved by 10% SDS-PAGE, followed by Western blotting with antiphosphorylated GSK-3α/β antibody. For analysis of the total amount of Akt, 40 μg of protein from the lysate samples are resolved by 10% SDS-PAGE, followed by Western blotting with anti-Akt antibody. |
Cell experiment: | All three prostate cancer cell lines (LNCaP, C4-2b, and PC-3 cells) are seeded at a density of 3 × 103 cells per well in 96-well microtiter culture plates. After overnight incubation, the medium is removed and replaced with a fresh medium containing different concentrations of ABT-627 (0-50 μM) diluted from a 10-mM stock. After 72 h of incubation with drug, 20 μL of MTT solution (5 mg/mL in PBS) are added to each well and incubated further for 2 h. Upon termination, the supernatant is aspirated and the MTT formazan formed by metabolically viable cells is dissolved in isopropanol (100 μL). The plates are mixed for 30 min on a gyratory shaker, and the absorbance is measured at 595 nm on a plate reader. |
Animal experiment: | YM598 (0.3, 1, and 3 mg/kg), atrasentan (0.3, 1, and 3 mg/kg), or 0.5% methyl cellulose as vehicle is orally administered to rats with a dosing cannula. Dosing volume of the test substances and vehicle is set at 5 mL/kg. Approximately 20 min after administration of compounds, the rats are anesthetized with sodium pentobarbital, and then pithed and ventilated 30 min after dosing. Approximately 1 h after oral administration of compounds, big endothelin-1 (1 nmol/kg) is intravenously administered, and blood pressure is measured. In these two experiments, the dose of test compound that cause 50% inhibition (ID50) of the big endothelin-1-induced increase in diastolic blood pressure is determined by linear regression analysis. |
References: [1]. Yuyama H, et al. Superiority of YM598 over atrasentan as a selective endothelin ETA receptor antagonist. Eur J Pharmacol. 2004 Sep 13;498(1-3):171-7. | |
| Cas No. | 195733-43-8 | SDF | |
| Chemical Name | (2R,3R,4S)-4-(1,3-benzodioxol-5-yl)-1-[2-(dibutylamino)-2-oxoethyl]-2-(4-methoxyphenyl)pyrrolidine-3-carboxylic acid;hydrochloride | ||
| Canonical SMILES | CCCCN(CCCC)C(=O)CN1CC(C(C1C2=CC=C(C=C2)OC)C(=O)O)C3=CC4=C(C=C3)OCO4.Cl | ||
| Formula | C29H39ClN2O6 | M.Wt | 547.08 |
| 溶解度 | DMSO : 28.57 mg/mL (52.22 mM; Need ultrasonic); H2O : 0.5 mg/mL (0.91 mM; ultrasonic and warming and adjust pH to 4 with HCl and heat to 60°C); 0.1 M HCL : < 1 mg/mL (insoluble) | Storage | Store at -20°C |
| General tips | Please select the appropriate solvent to prepare the stock solution according to the
solubility of the product in different solvents; once the solution is prepared, please store it in
separate packages to avoid product failure caused by repeated freezing and thawing.Storage method
and period of the stock solution: When stored at -80°C, please use it within 6 months; when stored
at -20°C, please use it within 1 month. To increase solubility, heat the tube to 37°C and then oscillate in an ultrasonic bath for some time. |
||
| Shipping Condition | Evaluation sample solution: shipped with blue ice. All other sizes available: with RT, or with Blue Ice upon request. | ||
| Prepare stock solution | |||

|
1 mg | 5 mg | 10 mg |
| 1 mM | 1.8279 mL | 9.1394 mL | 18.2789 mL |
| 5 mM | 0.3656 mL | 1.8279 mL | 3.6558 mL |
| 10 mM | 0.1828 mL | 0.9139 mL | 1.8279 mL |
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
 g
g
 μL
μL

Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
3. All of the above co-solvents are available for purchase on the GlpBio website.
Quality Control & SDS
- View current batch:
- Purity: >99.50%
- COA (Certificate Of Analysis)
- SDS (Safety Data Sheet)
- Datasheet
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *



















