Benazepril |
| カタログ番号GC11228 |
アンジオテンシン変換酵素阻害剤であるベナゼプリルは、高血圧の治療に使用される薬です。
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 86541-75-5
Sample solution is provided at 25 µL, 10mM.
Benazepril, an angiotensin converting enzyme inhibitor, which is a medication used to treat high blood pressure.Target: angiotensin converting enzyme (ACE)Benazepril is a medication used to treat high blood pressure (hypertension), congestive heart failure, and chronic renal failure. Upon cleavage of its ester group by the liver, benazepril is converted into its active form benazeprilat, a non-sulfhydryl angiotensin-converting enzyme (ACE) inhibitor [1].Animals were randomly divided into 4 groups: sham STNx group (control), STNx group, morning benazepril group (MB) and evening benazepril group (EB).Benazepril was intragastrically administered at a dose of 10 mg/kg/day at 07:00 and 19:00 in the MB group and EB group respectively for 12 weeks. All the animals were synchronized to the light:dark cycle of 12:12 for 12 weeks. Systolic blood pressure (SBP), 24-h urinary protein excretion and renal function were measured at 11 weeks. Blood samples and kidneys were collected every 4 h throughout a day to detect the expression pattern of renin activity (RA), angiotensin II (AngII) and aldosterone (Ald) by radioimmunoassay (RIA) and the mRNA expression profile of clock genes (bmal1, dbp and per2) by real-time PCR at 12 weeks. Our results showed that no significant differences were noted in the SBP, 24-h urine protein excretion and renal function between the MB and EB groups. There were no significant differences in average Ald and RA content of a day between the MB group and EB group. The expression peak of bmal1 mRNA was phase-delayed by 4 to 8 h, and the diurnal variation of per2 and dbp mRNA diminished in the MB and EB groups compared with the control and STNx groups. It was concluded when the similar SBP reduction, RAAS inhibition and clock gene profile were achieved with optimal dose of benazepril, morning versus evening dosing of benazepril has the same renoprotection effects [2].Clinical indications: Congestive heart failure; End stage renal disease; HypertensionFDA Approved Date: Toxicity: headaches; cough; Anaphylaxis; angioedema; hyperkalemia
References:
[1]. Hou FF, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006 Jan 12;354(2):131-40.
[2]. Huang XM, et al. Effects of chronotherapy of benazepril on the diurnal profile of RAAS and clock genes in the kidney of 5/6 nephrectomy rats. J Huazhong Univ Sci Technolog Med Sci. 2013 Jun;33(3):368-74.
| Cas No. | 86541-75-5 | SDF | |
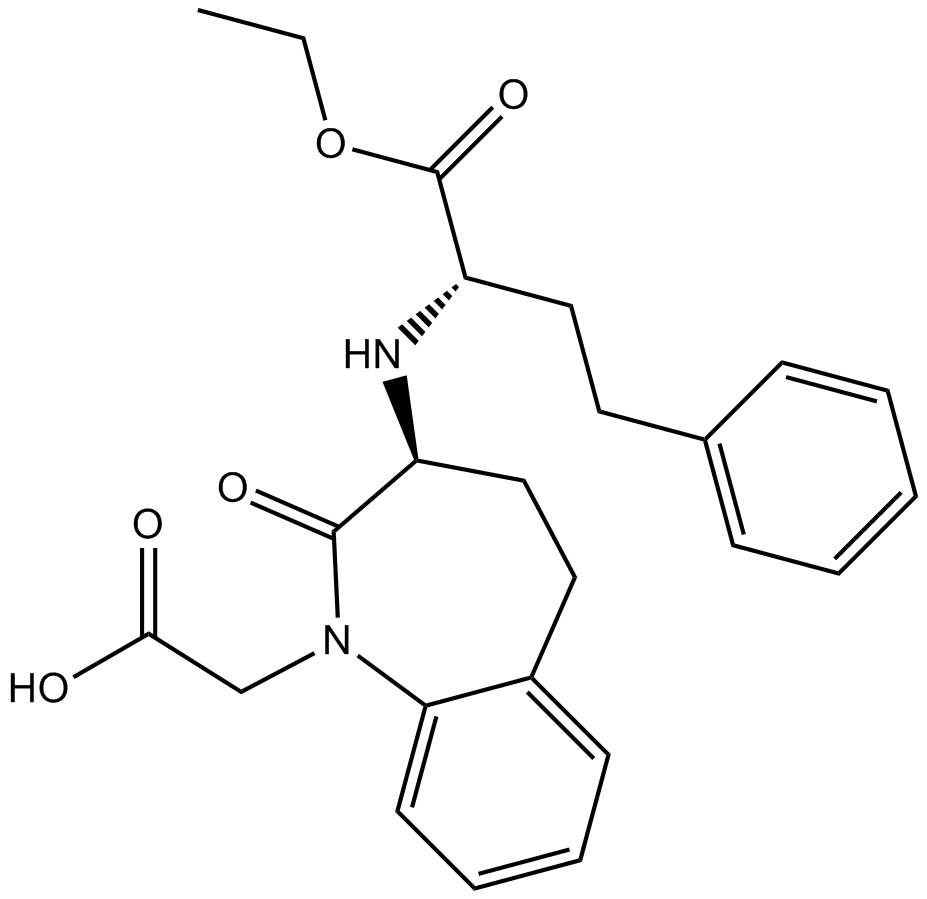
| Chemical Name | 2-[(3S)-3-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]-2-oxo-4,5-dihydro-3H-1-benzazepin-1-yl]acetic acid | ||
| Canonical SMILES | CCOC(=O)C(CCC1=CC=CC=C1)NC2CCC3=CC=CC=C3N(C2=O)CC(=O)O | ||
| Formula | C24H28N2O5 | M.Wt | 424.49 |
| 溶解度 | DMSO : 85mg/mL | Storage | Store at -20°C |
| General tips | Please select the appropriate solvent to prepare the stock solution according to the
solubility of the product in different solvents; once the solution is prepared, please store it in
separate packages to avoid product failure caused by repeated freezing and thawing.Storage method
and period of the stock solution: When stored at -80°C, please use it within 6 months; when stored
at -20°C, please use it within 1 month. To increase solubility, heat the tube to 37°C and then oscillate in an ultrasonic bath for some time. |
||
| Shipping Condition | Evaluation sample solution: shipped with blue ice. All other sizes available: with RT, or with Blue Ice upon request. | ||
| Prepare stock solution | |||

|
1 mg | 5 mg | 10 mg |
| 1 mM | 2.3558 mL | 11.7788 mL | 23.5577 mL |
| 5 mM | 0.4712 mL | 2.3558 mL | 4.7115 mL |
| 10 mM | 0.2356 mL | 1.1779 mL | 2.3558 mL |
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
 g
g
 μL
μL

Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
3. All of the above co-solvents are available for purchase on the GlpBio website.
Quality Control & SDS
- View current batch:
- Purity: >98.00%
- COA (Certificate Of Analysis)
- SDS (Safety Data Sheet)
- Datasheet
Average Rating: 5 (Based on Reviews and 3 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *



















