N6-Methyladenosine-5'-Triphosphate |
| カタログ番号GN20007 |
N6-メチルアデノシン(N6-メチルATP)は、アデノシンの塩基修飾された類似体であり、天然RNA中に少量存在する核酸塩基です(Meyer et al.)。
Products are for research use only. Not for human use. We do not sell to patients.
Sample solution is provided at 25 µL, 10mM.
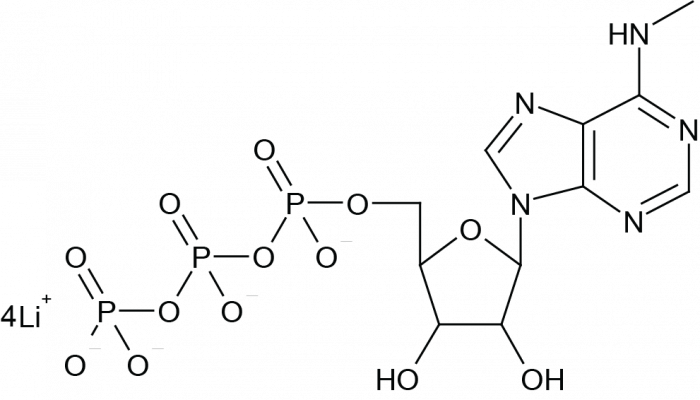
N6-Methyladenosine-5-Triphosphate (N6-methyl ATP; m6ATP) is a base-modified analog of adenosine, identified as a minor nucleoside in natural RNA[1]. N6-Methyladenosine-5’-Triphosphate is a dynamic, reversible, covalent modification of ribonucleotides, commonly found in eukaryotic messenger RNA (mRNA), and plays a crucial regulatory role in RNA splicing, export, stability, and translation[2, 3]. N6-Methyladenosine-5-Triphosphate serves as a substrate for RNA polymerase and is used as a complete substitute for ATP in mRNA modification reactions[4, 5]. Additionally, N6-Methyladenosine-5-Triphosphate is an effective agonist for P2Y purinergic receptors in guinea pigs and Escherichia coli[6]. This product is provided in the form of a lithium salt solution.
References:
[1] Yuan Y, Arneson R, Burke E, et al. Efficient 3′-end tailing of RNA with modified adenosine for nanopore direct total RNA sequencing[J]. bioRxiv, 2024: 2024.02. 24.581884.
[2] Zheng H, Li S, Zhang X, et al. Functional implications of active N6-methyladenosine in plants[J]. Frontiers in Cell and Developmental Biology, 2020, 8: 291.
[3] Anderson S J, Kramer M C, Gosai S J, et al. N6-methyladenosine inhibits local ribonucleolytic cleavage to stabilize mRNAs in Arabidopsis[J]. Cell reports, 2018, 25(5): 1146-1157. e3.
[4] Parr C J C, Wada S, Kotake K, et al. N 1-Methylpseudouridine substitution enhances the performance of synthetic mRNA switches in cells[J]. Nucleic Acids Research, 2020, 48(6): e35-e35.
[5] Potapov V, Fu X, Dai N, et al. Base modifications affecting RNA polymerase and reverse transcriptase fidelity[J]. Nucleic Acids Research, 2018, 46(11): 5753-5763.
[6] Burnstock G, Fischer B, Hoyle C H V, et al. Structure activity relationships for derivatives of adenosine‐5′‐triphosphate as agonists at P2 purinoceptors: Heterogeneity within P2x and P2y subtypes[J]. Drug development research, 1994, 31(3): 206-219.
| purity | >99.00% | Extinction Coefficient | 15,567 Lmol-1cm-1 at 265 nm |
| Formula | C11H18N5O13P3 (free acid) | M.Wt | 521.20 g/mole (free acid) |
| Salt Form | Li+ | 濃度 | 100 mM |
| Buffer | H2O | Storage | -20°C or below |
| 同義語 | N6-Methyl-ATP, m6ATP | Backbone | 5'-Triphosphate |
| Base Analog | Adenosine | Sugar Type | RNA |
| Nucleotide Category | Base Modified RNA | ||
| 応用 | Aptamers, Epigenetics/DNA Damage, In vitro Transcription, Mutagenesis, Photocrosslinking Studies | ||
Average Rating: 5 (Based on Reviews and 18 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *


















