GDC-0941 dimethanesulfonate (Synonyms: GDC-0941 (2 MeSO3H salt);GDC0941 dimethanesulfonate;GDC-0941;GDC0941;GDC 0941) |
| カタログ番号GC16154 |
クラスI PI3Kアイソフォームのパン阻害剤
Products are for research use only. Not for human use. We do not sell to patients.
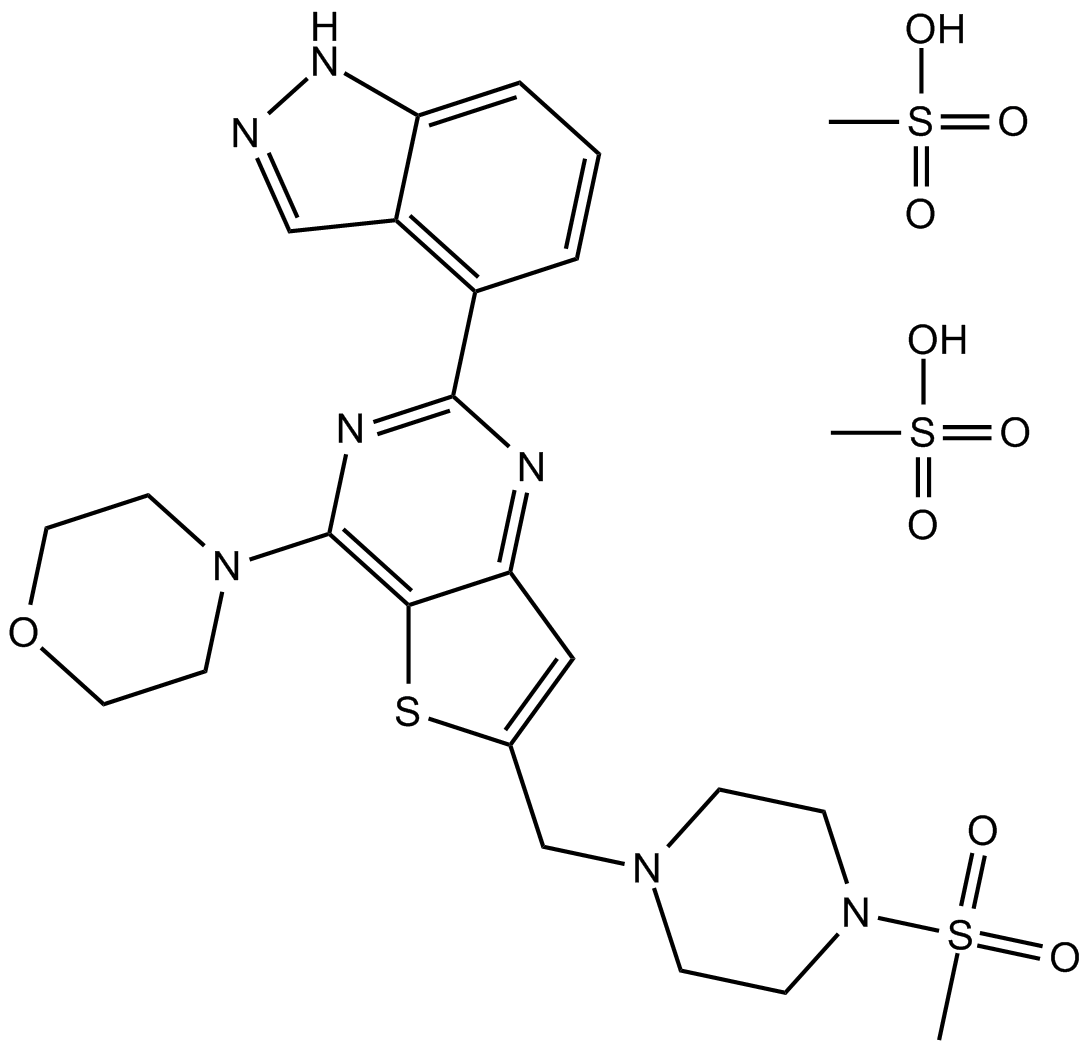
Cas No.: 957054-33-0
Sample solution is provided at 25 µL, 10mM.
GDC-0941 is a novel selective class I phosphatidylinositol-3-kinase (PI3K) inhibitor. Activation of PI3K/Akt signaling pathway is frequently associated with tumorigenesis. Deregulation of this pathway occurs frequently with a variety of cancers and may contribute to the resistance to many anticancer agents. [1] Developing novel small molecules that specifically block the PI3K/Akt pathway may inhibit tumor growth. GDC-0941 is designed to bind the ATP-binding pocket of PI3K and to prevent formation of phosphatidylinositol-3, 4, 5-triphosphate (PIP3), a second messenger that transmits PI3K downstream signals. [2, 3] It binds to PI3K in an ATP-competitive manner.
GDC-0941 is a potent small-molecule thieno [3, 2-d] pyrimidine inhibitor of the class I PI3K. It is highly selective against isoforms p110( and p110( with IC50 of 3 nM, and moderately selective against isoforms p110( and p110( with IC50s of 33 nM and 75 nM, respectively.
GDC-0941 inhibits cell proliferation in vitro and in vivo. It causes growth inhibition in a variety of cancer cell lines, including A2780, MDA-MB-361, PC3, and U87MG. [2] It also inhibits the growth of trastuzumab–sensitive and –resistant HER2-amplied cancer cells which harbor p110( mutations or PTEN loss. [4] GDC-0941 also reduces tumor volume in different xenograft models. [4]
GDC-0941 can be taken orally.
References:
[1]Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497-5510.
[2]Folkes AJ, Ahmadi K, Alderton WK, et al. The identification of 2-(1H-Indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008; 51: 5522-5532.
[3]Knight ZA, Shokat KM. Chemically targeting the PI3K family. Biochem Soc Trans. 2007; 35: 245-249.
[4]Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Br J Cancer. 2011; 104(7): 1116-25.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















