Anagrelide HCl (Synonyms: BL 4162A, BMY 26538-01) |
| Catalog No.GC11273 |
A PDE3 inhibitor
Products are for research use only. Not for human use. We do not sell to patients.
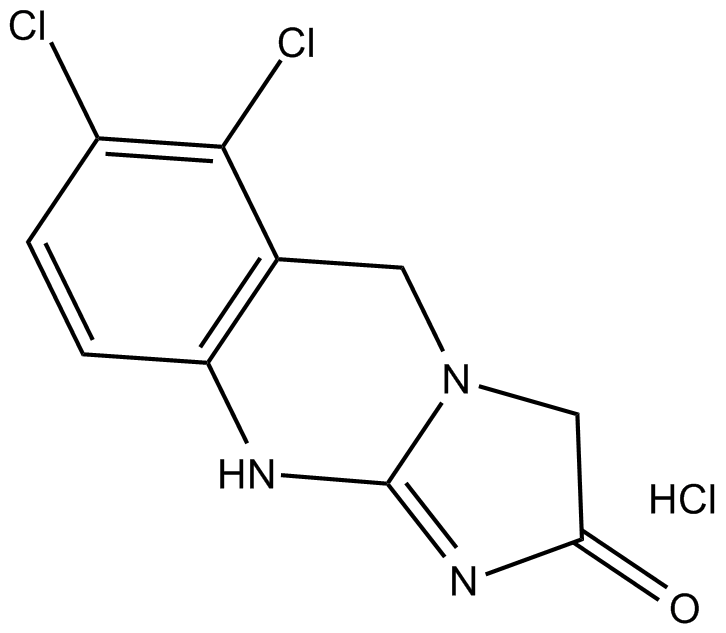
Cas No.: 58579-51-4
Sample solution is provided at 25 µL, 10mM.
Anagrelide is a selective thrombocytopenic agent [1].
Anagrelide is a FDA-approved drug for the treatment of essential thrombocythemia. It is originally identified as a potential inhibitor of platelet aggregation. Anagrelide selectively affects thrombocytes while shows no significant effect on white blood cells, erythrocytes, or coagulation. Anagrelide is revealed to be available in 0.5mg capsules for oral administration.
Anagrelide is a potent inotropic agent for dogs with remarkable vasodilatory activity. Additionally, anagrelide can reduce renal blood flow. Furthermore, anagrelide has shown to play roles in other chronic myeloproliferative disorders, such as polycythemia vera, chronic myeloid leukemia and agnogenic myeloid metaplasia. Anagrelide has some side effects involving headache, diarrhea, edema, palpitations, and abdominal pain [1].
References:
[1] Oertel MD. Anagrelide, a selective thrombocytopenic agent. Am J Health Syst Pharm. 1998 Oct 1;55(19):1979-86.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















