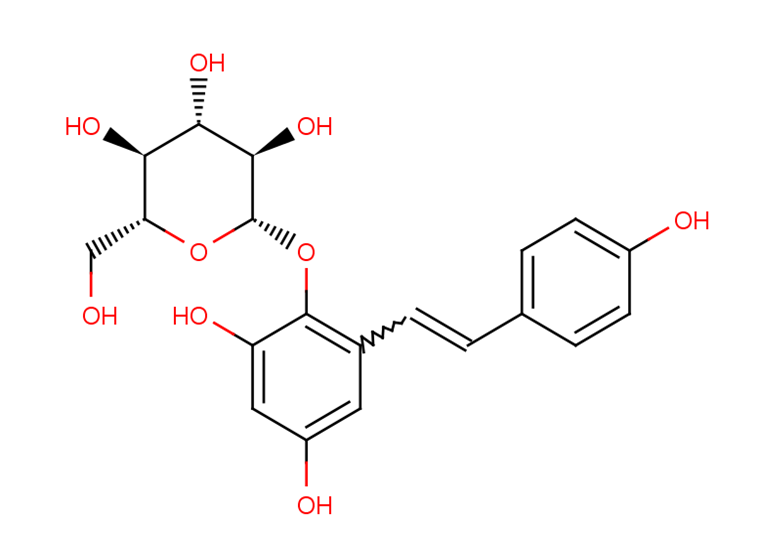
2,3,5,4′- tetrahydroxystilbene-2-O-β-D- glucopyranoside (TSG)
2,3,5,4′- tetrahydroxystilbene-2-O-β-D- glucopyranoside (TSG), identified as the most prevalent bioactive constituent derived from Polygonum multiflorum (Heshouwu), a traditional Chinese herb. TSG is a polyphenol compound that implies numerous antioxidative, anti-aging, anti-inflammatory, anti-hypercholesterolemic, hepatoprotective, and anti-tumor aspects of pharmacological actions. TSG was primarily found to be distributed in the liver after oral administration by investigating the distribution of Polygonum multiflorum characteristic elements in animal models. Moreover, comparing treated PM with raw PM indicates that raw PM exhibits much more severe hepatotoxicity than treated one, assuming that a higher concentration of TSG in raw PM probably be a toxic constituent of PM.
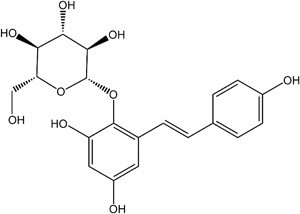
Figure 1: Chemical structure of 2,3,5,4′- tetrahydroxystilbene-2-O-β-D- glucopyranoside (TSG)
Prior studies suggested that TSG exerts anti-hyperlipidemia, anti-atherosclerosis, anti-cancer, and hepatoprotection attributes, guarding PC12 cells against apoptosis, initiating PI3K/Akt for stimulation of murine pre–osteoblastic MC3T3-E1 proliferation of cells and differentiation. Meanwhile, it is yet unknown exactly which element of Polygonum multiflorum causes toxicity or damages the liver, and how TSG acts as a hepatoprotective agent to attenuate liver injury under prediabetes. The main objective of this study is to analyze the effects of TSG on the immune system in cases of PM idiopathic hepatotoxicity, explicating viable T-cell activation mechanisms for the assessment of TSG’s influence on T lymphocytes under in vivo and in vitro conditions while offering manifestation for the toxic elements or mechanisms of PM-induced deterioration of the liver.
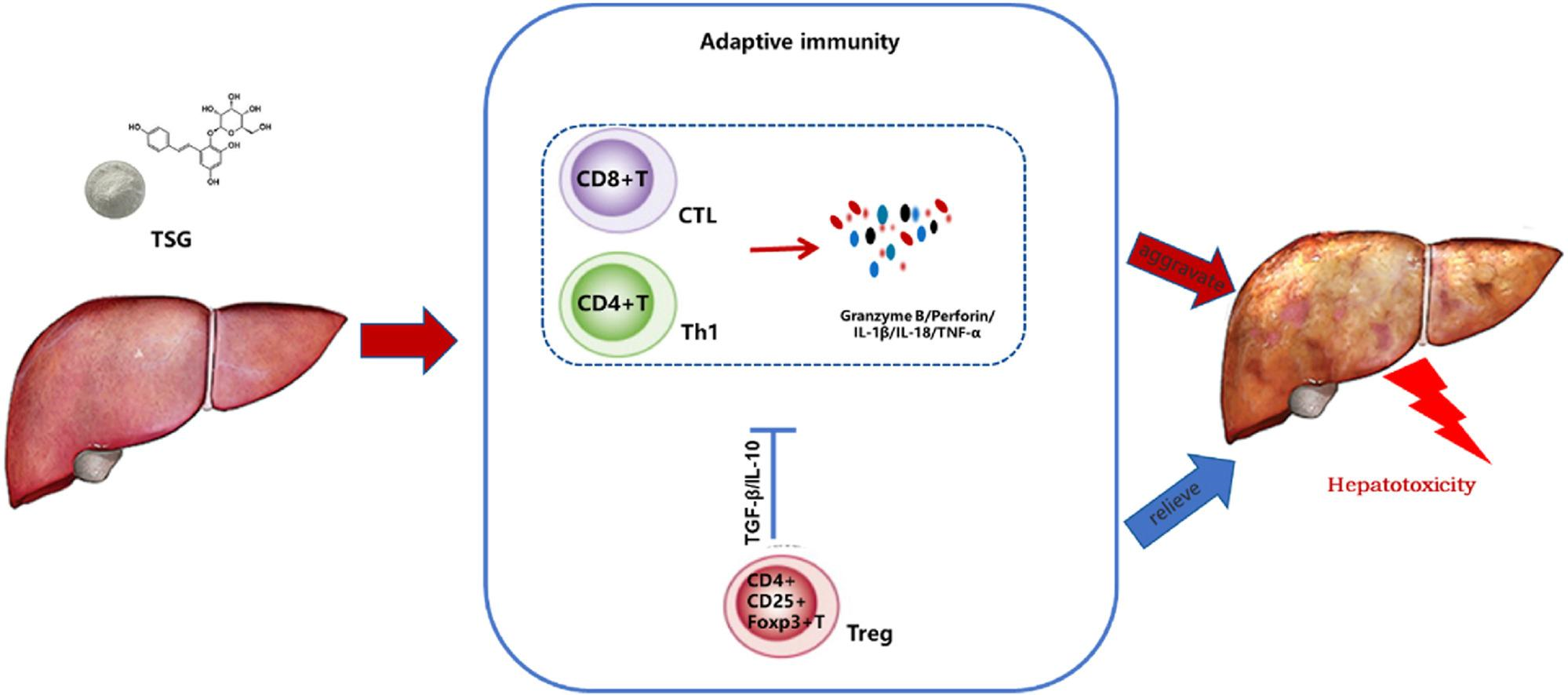
Figure 2: The mechanism of action of TSG-induced liver injury
TSG treated mild liver injury progression in mice :The mice were offered oral dosages of TSG in vivo at 100 mg/kg and 400 mg/kg/day for four weeks to resolve the influence of TSG on the liver while exhibiting the serum biochemical indexes, ALT or AST (Figure 3A, B). A substantial elevation in the TSG group was noticed in ALT/AST (*P < 0.05, **P < 0.01) depicting the establishment of inflammatory cell infiltration in the mice liver which suggests that TSG may influence mild damage to the liver from the TSG treated groups (Figure 3C).
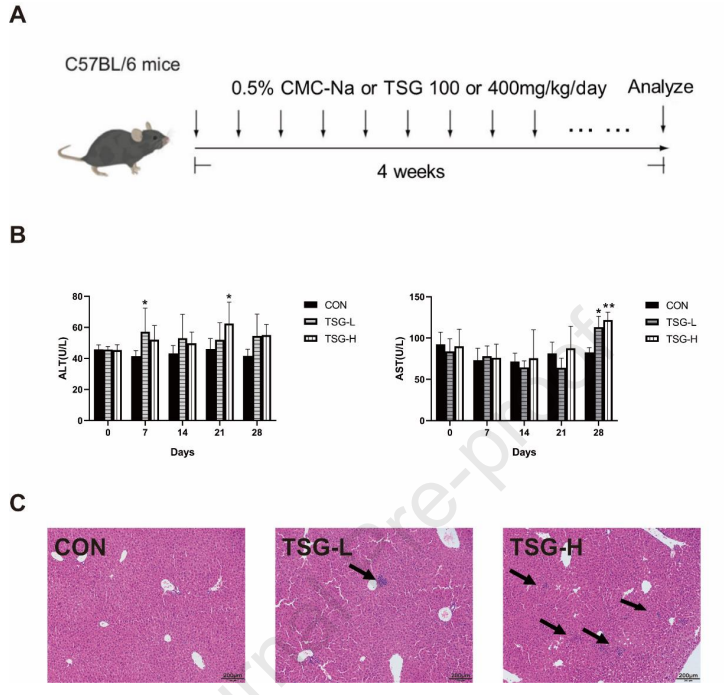
Figure 3: TSG-induced mild liver injury
TSG-stimulated immune cell activation in the liver :T lymphocytes perform an indispensable role in liver injury caused by idiosyncratic drugs, implementing FCM to determine TSG alterations in T cell populations of the mice liver. A leukocyte activation marker, CD69 is involved in the activation and proliferation of T cells, indicating the TSG-H group with significantly higher CD4+ T cell proliferation, CD69 expression on CD4+ T and CD8+ cells while comparing with the control. Proinflammatory cytokine levels in the serum and liver amplified by TSG :By upgrading the inflammatory reactions in drug-initiated damage, drug-induced hepatotoxicity may heighten the susceptibility due to alterations in cytokine levels. The TSG-treated group, such as serum TNF-α, IFN-γ, granzyme B, and perforin of inflammatory cytokine alterations is found to be significantly raised (Figure 4A). Meanwhile, the TSG administration led to a substantial elevation in IL-18 and TNF-α mRNA levels the rising tendencies in the expression of IL1β, granzyme B, and perforin were monitored (Figure 4B), suggesting the association between proinflammatory cytokine T cell proliferation and liver injury prompted by TSG.
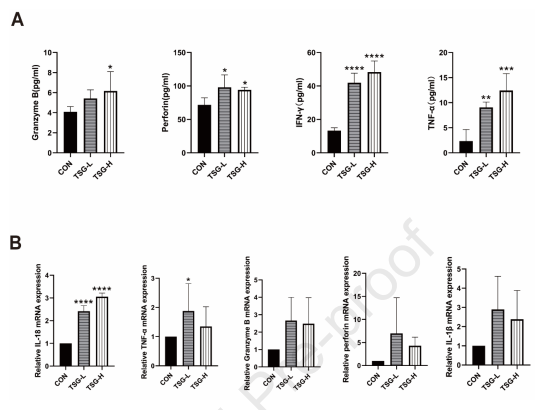
Figure 4: The pro-inflammatory cytokines levels in the liver and serum were upregulated by TSG.
TSG-induced liver injury transcriptomics interpretations :An RNA sequence assay was employed to demonstrate the differences in gene expression patterns of mouse liver tissues between TSG-treated groups and the control, investigating further the capable mechanisms associated with TSG-induced liver injury. A total of 591 differentially expressed genes (DEGs) were found of which 377 genes were upregulated and 214 were downregulated (Figure 5A), which were then examined for pathway enrichment to display the functional annotations of DEGs through the KEGG (Kyoto Encyclopedia of Genes and Genomes) and GO (Gene Ontology) (Figure 5B). TSG-induced liver injury is indicated to be discreetly associated with the immune system by embracing neutrophil extracellular traps, cell adhesion molecules, and chemokine signaling pathways based on KEGG pathway analysis. In GO enrichment analysis, activation of leukocytes concerning inflammatory response, leukocyte-cell adhesion, migration of mononuclear cells, control of the generation of interleukin-1 beta, and antigen presentation or processing were consistently validated. The eminent impact of TSG on pathways of Th1 and Th2 cell development and antigen processing or presentation offers a critical role of T cell-mediated adaptive immune response in TSG-induced hepatotoxicity. The immunological response to TSG-treated cells was characterized by implementing transcriptomics which indicates that in TSG-induced liver injury immune system activation was an indispensable element.
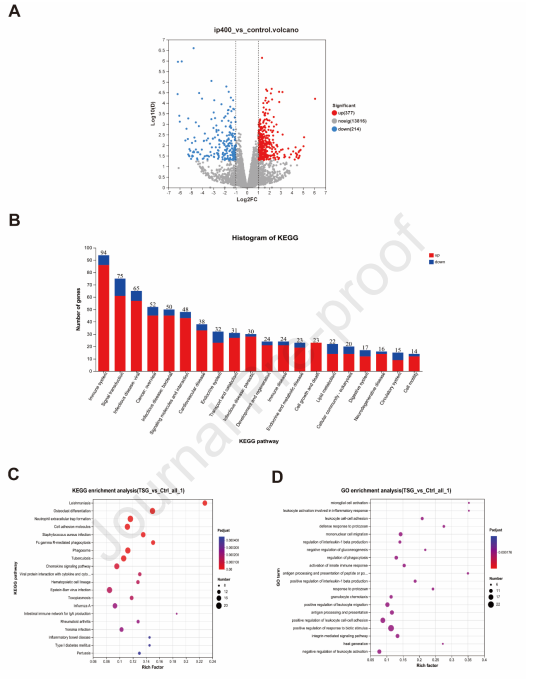
Figure 5: Implying transcriptomic analysis on liver tissues in mice treated 400 mg/kg/day and control
TSG derived from drug-naive mice in vitro activates T lymphocytes :By employing FCM, CCK8 tests, and ELISA, cell proliferation and secretion of immune molecules were assessed to analyze the direct effects of TSG on splenocytes in vitro (Figure 6A), exhibiting dose-dependent proliferation of TSG-induced splenocytes (Figure 6B).
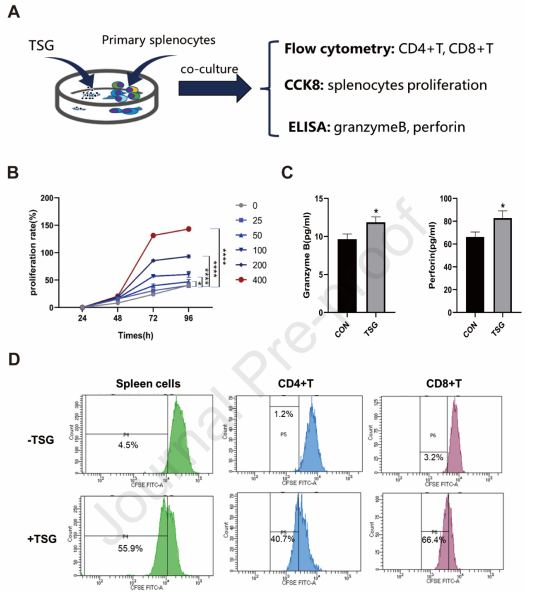
Figure 6: In vitro activation of T cells from drug-naive mice by TSG .The evaluation of T cell proliferation was done by implementing flow cytometry (FCM) after tagging splenocytes with CD3/CD4/CD8 antibodies and CFSE (BD Biosciences) treated with or without TSG for 72 hours, where TSG augmented proliferation in CD4+T and CD8+T cells (Figure 6C, D). Meanwhile, the granzyme B and perforin concentrations in cell culture supernatant were analyzed by ELISA along with TSG, uncovering a potential association between the stimulatory impact of TSG-induced hepatotoxicity and cytotoxic secretion of immune molecules.
The PM-induced liver injury involved ordinary idiosyncratic drug reactions, suggesting the interaction of medications or their metabolites with the host immune system. By a clinical investigation, activation of Kupffer cell and inflammatory cell infiltration were significantly observed in Journal Pre-proof PM-DILI patients while the precise toxic constituents of PM are still unclear in light of the complexity and variability of their chemical makeup. TSG being the primary bioactive component makes up around 2~6% of PM's total chemical composition and is the quantitative indication of inducing liver injury. Meanwhile, the research studies indicate that TSG enormously contributes to the hepatotoxicity of PM by modifying CYP gene expression to encourage autophagy, upsetting the homeostasis of bile acid, and phospholipid efflux.
This study suggests that TSG exhibits significant involvement in the idiosyncratic hepatotoxicity of PM along with sole TSG or conjugating anti-CD25 did not induce an elevation in the levels of ALT/AST in the blood of mice; nevertheless, HE staining uncovered a considerable increase in inflammatory cell infiltration in the liver. Moreover, the levels of granzyme B and IFN-γ in the serum display a marked increase by TSG conjugated with anti-CD25 in contrast to sole TSG while the lack of baseline illnesses in the animal model of PM hepatotoxicity is one of its encumbrance. In a hepatic tolerogenic milieu, the inadequacy of pathogen-associated and danger-associated molecular patterns brought on by immunological stress conditions restrict the development of severe liver inflammation and injury through TSG-driven immune activation hampering. The critical role of TSG-induced T-cell activation and production of inflammatory cytokines was demonstrated through ongoing investigations in TSG-driven hepatotoxicity, indicating activation of the immune system to initiate inflammatory reactions and liver damage caused by TSG. This research designated the basic framework or mechanisms of PM to incite liver injury and TSG-induced immune system activation association with toxic pathways or processes.












Comments