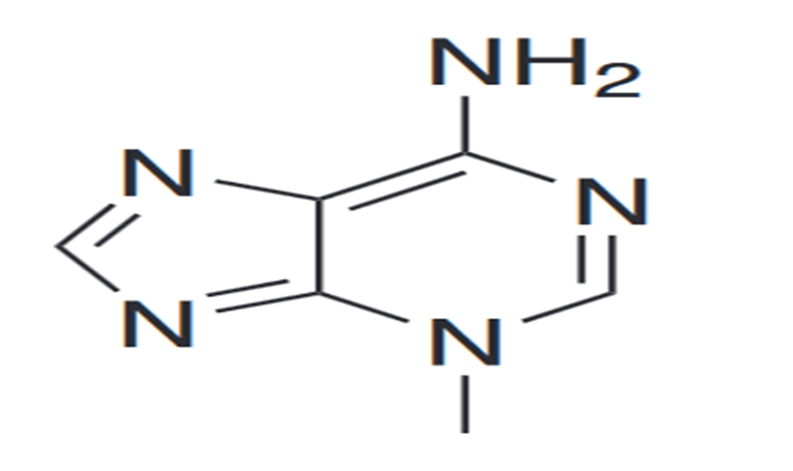
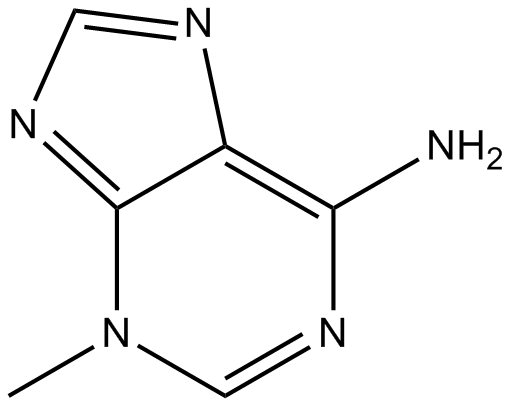
3-Methyladenine
By searching deep in the field of cellular biology by using the microscopic aspects, we revealed the surprising molecule under the name of 3-Methyladenine (3-MA), a compound that does not have its recognization under the heading of DNA or proteins, but play a vital role in the front line of cellular processes. This finding is not just a compound or molecule but it works like a key to open the locks of the mystery full world of cellular autophagy and it’s implications to human health and disease. In the world full of scientific discovery, this compound provide us insights into the process of autophagy, where cells for the sake of self-safety and self-preservation chopped down it’s own components. This process, as it proceeds for the sake of maintenance of cellular health and to create the balance, is essential for the clearance of cellular debris, just like as the waste management system works in the boundary of the city. When this debris clearing system, autophagy, does not processed properly then a series of disease like cancer, neurodegeneration and infections are ensured.
3-Methyladenine (under the abbreviatory name of 3-MA) impose any inhibitory effects on phosphatidylinositol 3-kinases (PI3K). PI3K is a front line player in many biological processes. Like the control for the activation of mTOR, major player for autophagy. It is a process in which the cytoplasmic components like organelles and pathogens are packed in a double-membrane-bound, auto phagosome and passed to the lysosome for destruction. 3-Methyladenine has an inhibitory effects on autophagy by blocking the formation of auto phagosome by inhibiting the complex of class lll PI3K. Interestingly, 3-methyladenine appears to play a dual role in autophagy. Prolonged treatment with 3-methyladenine promotes autophagy under nutrient-rich conditions, whereas 3-methyladenine inhibits starvation-induced autophagy. These results are attributed to differential effects on class I versus class III PI3K. It blocks class I PI3K persistently, whereas its suppressive effect on class III PI3K is transient. In addition to its role in autophagy, 3-methyladenine has been implicated in cancer therapy. It has been revealed that 3-methyladenine suppresses the invasion of highly metastatic cancer cells through the inhibition of class I and II PI3K. 3-Methyladenine can also induce caspase-dependent cell death that is independent of autophagy inhibition.
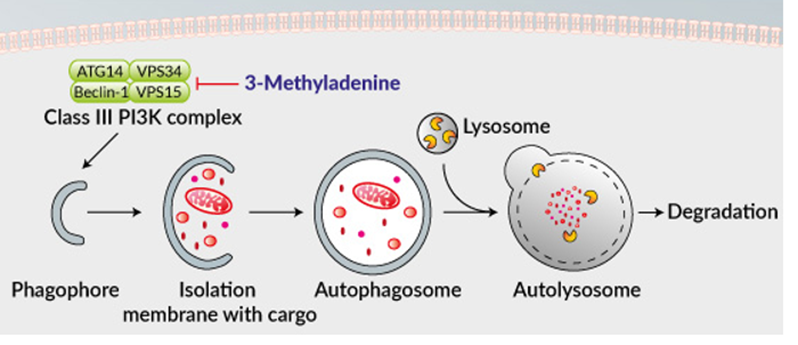
Figure 1: Inhibition of PI3K -III complex and autophagy by 3-methyladenine.
To understand the link between Ca2+ entry via the TRPC1 channel and autophagy, 3-methyladenine (3-MA) a known autophagy inhibitor,4 was used. The cells were pretreated with 1 mM 3-MA, along with DMOG, or DFO, or serum-free media, and protein expression of STIM1, Orai1, TRPC1, and autophagy markers were analyzed. Importantly, cells treated with 3-MA showed no increase in DMOG or serum-deprived increase in TRPC1 expression (Figure 2a). Furthermore, no increase in beclin-1 an autophagic marker was observed in cells pretreated with 3-MA along with DMOG- or serum-deprived conditions (Figure 2a). Pretreatment with 3-MA also attenuated the increase in Ca2+ entry that was induced by DMOG and serum starvation in both SHSY-5Y (Figures 2b and c) and HSG cells (result not shown). Consistent with these results, pretreatment with 3-MA also attenuated cell viability (Figure 2d) and serum starvation-induced increase in Tg-induced Ca2+ currents (Figures 2e and f). Altogether, these data suggest that Ca2+ entry via the TRPC1 channel is essential for autophagy that leads to the inhibition of cell death and loss of TRPC1 function, or autophagy could lead to a decrease in cell survival.
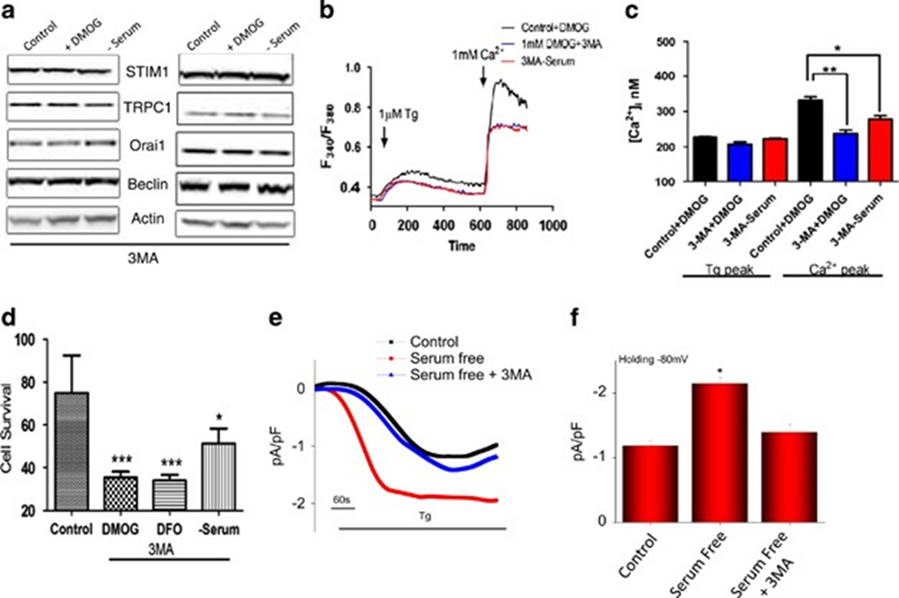
Figure 2: Pretreatment with autophagy inhibitor 3-methyladenine (3-MA) attenuated the intracellular calcium influx and induced apoptosis.
Pretreatment with autophagy inhibitor 3-methyladenine (3-MA) attenuated the intracellular calcium influx and induced apoptosis. (a) Western blot images showing the expression of SOCE components STIM1, TRPC1, and Orai1, autophagy marker beclin-1, and loading control actin in HSG and SHSY-5Y cells pretreated with 1 mM DMOG or in serum-free media in the presence of 1 mM autophagy marker 3-MA for 24 h. (b) Representative traces showing the transient increase in [Ca2+]i after the addition of 1 mM calcium in the presence of 1 mM 3-MA to SHSY-5Y cells pretreated with 1 mM DMOG or in serum-free media. (c) The bar diagram shows the [Ca2+]. In nM concentration of the above-mentioned experiment. Each bar gives the mean±S.E.M. of 50 separate experiments. *P<0.05, **P<0.01. (d) Bar diagram showing the cell viability assay (MTT assay) in the SH-SY5Y cells, pretreated with 1 mM DMOG in the presence of 1 mM. Each bar gives the mean±S.E.M. of four separate experiments. ***P<0.001. (e) Application of 1 μM Tg in bath solution induced inward currents at −80 mV in control, cells treated in serum-free media, and autophagy inhibitor 3-MA-treated cells. (f) Average (8–10) recordings with current intensity at −80 mV are shown, *P<0.05.
To further determine the role of autophagy induction in mediating the protective effect of metformin in cisplatin -induced tubular cell death, we used 3-methyladenine (3-MA) to suppress autophagy in NRK-52E cells. Western blotting assay showed that metformin could induce LC3-II formation. As expected, 3-MA could largely abolish LC3-II induction (Fig. 3A). We then detected cleaved caspase 3 in NRK-52E cells with western blotting assay and found that cisplatin-induced caspase 3 cleavage was largely abolished by metformin treatment, whereas 3MA could restore the reduction of caspase 3 cleavage in NRK-52E cells treated with metformin plus cisplatin (Fig. 3B, C). TUNEL staining further confirmed the results of the western blotting assay (Fig. 3D,E). These results suggest that autophagy induction is required for metformin to protect against cisplatin-stimulated tubular cell apoptosis.
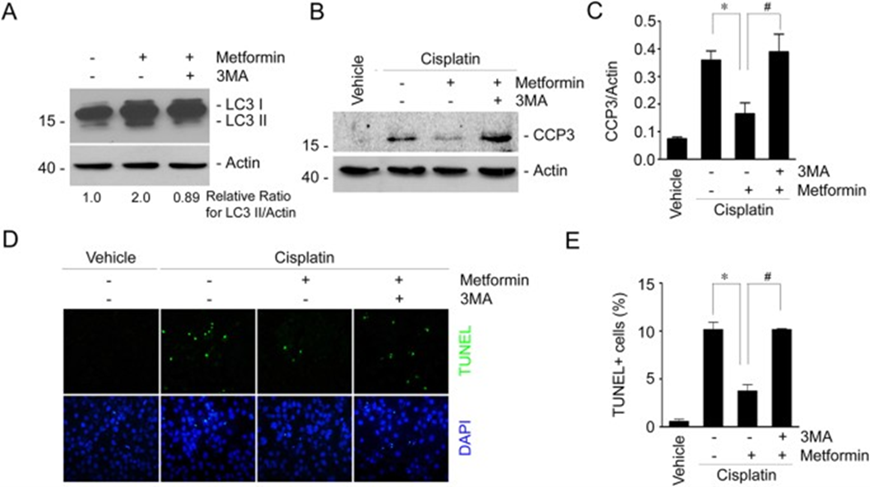
Figure 3: Inhibition of autophagy by 3-methyladenine diminishes the protective effect of metformin in cisplatin-induced cell apoptosis in NRK-52E cells.
(A) Western blot assay showing the LC3-II abundance in NRK-52E cells. NRK-52E cells were incubated with metformin in the absence or presence of 3MA (10 mM) for 12 h. The gels were run under the same experimental conditions. (B) Western blot assay showing caspase 3 cleavage in NRK-52E cells. The gels were run under the same experimental conditions. (C) The graphs show the semi-quantitative analysis results for the abundance of cleaved caspase 3 protein in NRK-2E cells. *P < 0.05 compared to the cells treated with cisplatin alone (n = 4); #P < 0.05 compared to the cells treated with cisplatin plus metformin (n = 4). (D) Representative micrographs showing the TUNEL staining among different groups as indicated. (E) The graphs show the quantitative determination of TUNEL-positive cells among different groups. Data are presented as the percentage of the TUNEL-positive cells. *P < 0.05 compared to the cells treated with cisplatin alone (n = 3); #P < 0.05 compared to the cells treated with cisplatin plus metformin (n = 3).
This small molecule is reported to inhibit autophagy and apoptosis. The ability of 3-methyladenine to suppress the formation of electron microscopically visible auto phagosomes suggests that it may be regarded as a specific inhibitor of autophagy.














Comments