Atezolizumab (MPDL3280A)
Atezolizumab is an Fc-engineered , humanized, monoclonal antibody that targets programmed death ligand 1 (PD-L1). Atezolizumab prevents the binding of PD-L1 to receptors programmed death 1 (PD-1) and B7. Atezolizumab is a non-glycosylated IgG1 kappa immunoglobulin that has molecular mass of 145 kDa.
We present a rare case of atezolizumab-induced autoimmune diabetes mellitus in a patient treated for metastatic breast cancer. While cases of ICI-induced autoimmune DKA have been previously reported related to the treatment of urothelial, skin, lung, and renal cancer, no prior case has been described in the setting of metastatic breast cancer treatment. Atezolizumab is a humanized monoclonal antibody to programmed death-ligand 1 (PD-L1) and is classified as an ICI. Atezolizumab is FDA-approved for the treatment of various types of cancer, including metastatic non-small cell lung cancer, locally advanced or metastatic urothelial carcinoma, and triple-negative breast cancer in patients who as an intravenous infusion every three weeks until disease progression or unacceptable toxicity is noted.4Atezolizumab functions by inhibiting the binding of programmed cell death 1 (PD-1), which is expressed on T cells, to PD-L1, which is expressed on antigen-presenting cells and tumor cells (Figure, A). This inhibition allows the T cells to activate and proliferate against tumor cells. Unfortunately, pancreatic islet cells also express PD-L1. Atezolizumab can inhibit the binding of PD-1 to PD-L1 and activate the T cell against the pancreatic islet cells. Subsequent destruction of pancreatic islet cells results in autoimmune-induced diabetes.
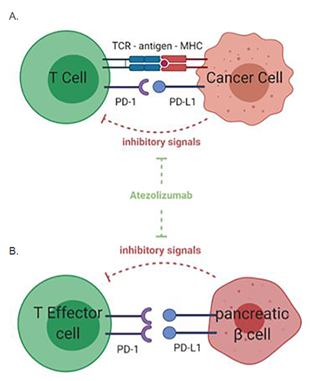
Figure 1: Mechanism of anti-PD-L1, atezolizumab, on T-cell response to cancer cells
PD-L1 is expressed in cancer cells and inhibits T-cell activation when binding to the PD-1 receptor on T-cell surface. Anti-PD-1/PD-L1 treatment with atezolizumab leads to the inhibition of this inhibitory pathway and subsequent enhanced T-cell activity against tumor cells. (B) Mechanism of action of anti-PD-L1 agent on immune tolerance in the pancreas. PD-1/PD-L1 interaction between effector T cells and pancreatic insulin-secreting β cells inhibits T-cell activation against the pancreas. The anti-PD-L1 agent, atezolizumab, blocks the PD-1/PD-L1 interaction, leading to activation of self-reactive T cells and destruction of the pancreatic islet cells of Langerhans. This results in immunotherapy-induced autoimmune diabetes.
To address the mechanisms of the action of atezolizumab in MDA-MB-231, we first examined the effect of atezolizumab on PD-L1 (protein and mRNA) expression. The surface expression of PD-L1 was determined by flow cytometric analysis. Almost all MDA-MB-231 cells (non-treated or treated with human IgG1 isotype control) were positive for PD-L1, however the detection of the PD-L1 epitope was blocked by the specific antibody, atezolizumab, after 24 h treatment (Figure 2A). RT-qPCR gene expression analysis showed that the PD-L1 mRNA expression level was not affected upon atezolizumab treatment, compared to non-treated cells and cells treated with isotype control (Figure 2B). The expression of PD-L1 mRNA level in the isotype-treated cells was similar to that of non-treated cells (Figure 2B). Additionally, data from western blots showed no difference in PD-L1 protein expression following atezolizumab treatment at 24 h (Figure 2C).
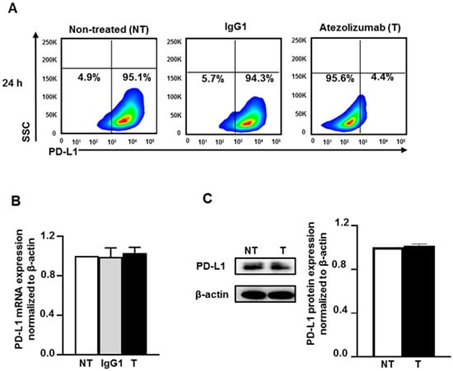
Figure 2: Atezolizumab blocks the Epitope of PD-L1 and does not alter the PD-L1 mRNA and protein expression
Effect of atezolizumab on PD-L1 expression in MDA-MB-231 cells. MDA-MB-231 cells were cultured without or with 0.5 µg/mL of human IgG1 (isotype control) or atezolizumab. The surface expression of PD-L1 was determined at 24 h post-treatment by flow cytometry. Representative flow cytometric plots show the percentage of cells expressing PD-L1 in non-treated, IgG1- and atezolizumab-treated cells (A). The expression of PD-L1 mRNA was determined by RT-qPCR (B). The expression of PD-L1 protein was determined by western blot and densitometric analysis (C). More detailed western blot information can be found. The relative expression of mRNA and protein were normalized to β-actin. Results are from two independent experiments and expressed as the mean ± SEM.
Collectively, these data indicate that atezolizumab blocks the epitope of PD-L1 without altering the expression of PD-L1 at both the mRNA and protein levels. It is most likely that atezolizumab blocks the detection of PD-L1 by the anti-PD-L1-APC antibody used for flow cytometry. Atezolizumab binding to PD-L1 at the cell surface would hamper the binding of the anti-PD-L1-APC antibody. It is worth noting that the isotype control does not affect the epitope of PD-L1, suggesting the specificity of atezolizumab in blocking PD-L1 antigen.
Next, we found that about 19% of the genes that were downregulated in atezolizumab-treated cells are associated with EMT, 33% are related to cell migration/invasion and metastasis, 16% are associated with signaling transduction, favoring cell proliferation and EMT, 5% are anti-apoptotic, 8% are related to cell growth and tumor cell proliferation, and 19% are associated with hypoxia (Figure 3A). Selected genes from both upregulated and downregulated panels, including CD40, MMP9, BCL2L15, and NDUFS3 are shown in Figure 3B—values below the linear regression line (as shown in red) indicate “downregulation” and above the line indicate an “upregulation”.
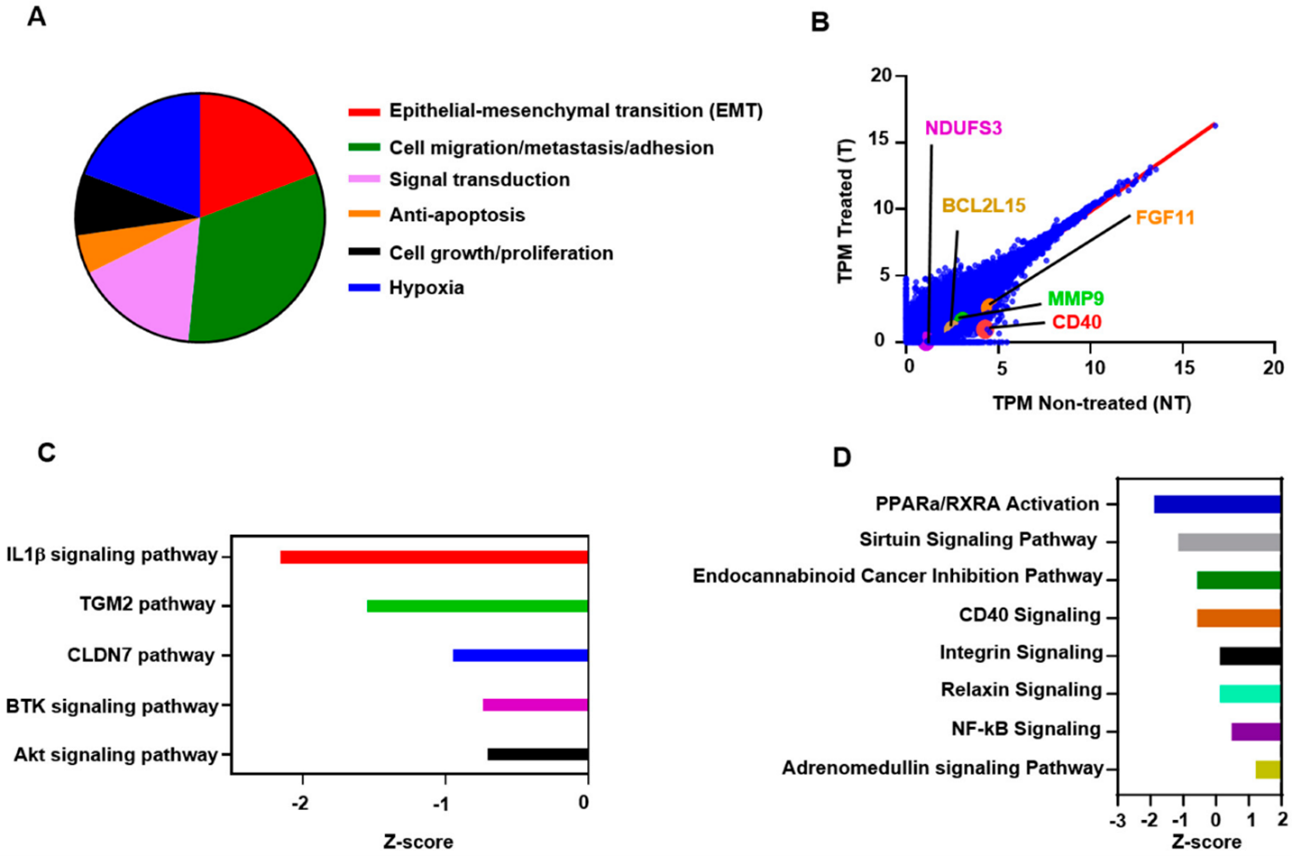
Figure 3: Atezolizumab Downregulates NF-kB, Akt, and CD40 Signaling Pathways
The mode of action of Atezolizumab and the involvement of the PD-L1 pathway in pediatric solid tumors: Chinese hamster ovary cells are used to manufacture atezolizumab, a humanized kappa immunoglobulin (Ig) G1 monoclonal antibody with two heavy chains (448 amino acids) and two light chains (214 amino acids). Through a single amino acid substitution (from asparagine to alanine) at position 298 on the heavy chain, atezolizumab was engineered to eliminate the Fc-effector function. This leads to a non-glycosylated antibody with minimal binding to Fc receptors, which in turn eliminates detectable Fc-effector function and depletes human cells expressing programmed death-ligand 1 (PD-L1). Targeting human PD-L1, atezolizumab prevents it from interacting with its receptors, B7.1, and programmed death-1 (PD-1), both of which can send signals to T cells that are suppressive. Cancer immune evasion is mediated by PD-L1 expression in tumors and immune cells; hence, blocking PD-L1 binding is a compelling tactic to reestablish tumor-specific T-cell immunity. Anti-PD-1 and anti-PD-L1 treatments have produced tumor responses in adult cancer patients with a variety of cancer types. Research on adult malignancies has revealed that tumor-intrinsic mechanisms, such as genomic amplification on TC or adaptive regulation of PD-L1 via IFNγ on IC, differently control PD-L1 expression on tumor cells (TC) and immune cells (IC).
Figure 4: Antigen-binding fragment of atezolizumab (blue) in complex with PD-L1 (pink)
The correlation between atezolizumab outcomes and PD-L1 expression has demonstrated that PD-L1 expression on immune cells is linked to a clinical advantage in bladder cancer, while PD-L1 expression on both TC and IC is linked to a clinical advantage in non-small cell lung cancer (NSCLC). Surprisingly, there seems to be a strong correlation between the biology of pre-existing immunity and clinical benefit in both NSCLC and bladder cancer, as indicated by the tumor IFNγ gene signature. Numerous pediatric tumor forms, such as high-grade glioma, rhabdomyosarcoma, non-Hodgkin's lymphoma, Hodgkin's lymphoma, soft tissue sarcoma, osteosarcoma, Ewing sarcoma, neuroblastoma, and Wilms' tumor, have been documented to express PD-L1 by malignant cells.













Comments