KX2-391 (Synonyms: KX 01, Tirbanibulin) |
| Catalog No.GC14288 |
KX2-391(KX2-391)은 Src의 펩타이드 기질 부위를 표적으로 하는 Src의 억제제이며, 암 세포주에서 GI50이 9-60nM입니다.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 897016-82-9
Sample solution is provided at 25 µL, 10mM.
KX2-391 is a highly selective inhibitor of Src kinase with IC50 value of 20nM [1].
KX2-391 is a non-ATP competitive inhibitor of Src. It is the first inhibitor that targets Src kinase within the substrate binding site. KX2-391 inhibits Src catalyzed trans-phosphorylation of FAK, Shc, paxillin as well as Src kinase autophosphorylation. KX2-391 has no effects on PDGFR, EGFR, JAK1, JAK2 and Lck demonstrating it as a selective inhibitor. It is also found to be an inhibitor of tubulin polymerization through binding to the unique confirmation on heterodimeric tubulin. In cellular assays, KX2-391 shows growth inhibition in NIH3T3/c-Src527F cells and SYF/c-Src527F cells with GI50 values of 23nM and 39nM, respectively [1, 2].
Since Src acts as a regulator in cell proliferation survival, motility and invasiveness, KX2-391 is potent against a variety of solid tumors and many leukemia tumors. It is shown to inhibit primary tumor growth and to suppress metastasis [2].
References:
[1] Fallah-Tafti A, Foroumadi A, Tiwari R, et al. Thiazolyl N-benzyl-substituted acetamide derivatives: Synthesis, Src kinase inhibitory and anticancer activities. European journal of medicinal chemistry, 2011, 46(10): 4853-4858.
[2] Naing A, Cohen R, Dy G K, et al. A phase I trial of KX2-391, a novel non-ATP competitive substrate-pocket-directed SRC inhibitor, in patients with advanced malignancies. Investigational new drugs, 2013, 31(4): 967-973.
| Cell experiment [1-3]: | |
|
Cell lines |
HCC cell lines Huh7, PLC/PRF/5, Hep3B, and HepG2 |
|
Preparation method |
The solubility of this compound in DMSO is >121mg/mL. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
6,564 to 0.012 nM, 3 days |
|
Applications |
KX2-391 showed dose-response curves against all four HCC cell lines Huh7, PLC/PRF/5, Hep3B, and HepG2 with the GI50 of 9 nM, 13 nM, 26 nM, and 60 nM, respectively. In NIH3T3/c-Src527F and SYF/c-Src527F cells, KX2-391 inhibited cell growth with the GI50 of 23 nM and 39 nM, respectively. KXO1 (10-30 nM) could halve proliferation rates (GI50) of a panel of human cancer cell lines known to have activated levels of SFK- such as HT-29 human colon cancer cells, as well as NIH3T3/c-Src527F cells. KXO1 inhibited anchorage-independent growth of HT-29 and 3T3/c-Src527F cells. |
| Animal experiment [3]: | |
|
Animal models |
nude mice bearing 50 cc HT-29 tumors |
|
Dosage form |
Oral administration, 5 mg/kg bid |
|
Application |
Treatment of nude mice bearing 50 cc HT-29 tumors with 5 mg/kg KXO1 bid p.o. resulted in a 70% reduction tumor growth, with no significant toxicity to the host as determined by weight loss. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Lau G M, Lau G M, Yu G L, et al. Expression of Src and FAK in hepatocellular carcinoma and the effect of Src inhibitors on hepatocellular carcinoma in vitro[J]. Digestive diseases and sciences, 2009, 54(7): 1465-1474. [2]. Fallah-Tafti A, Foroumadi A, Tiwari R, et al. Thiazolyl N-benzyl-substituted acetamide derivatives: synthesis, Src kinase inhibitory and anticancer activities[J]. European journal of medicinal chemistry, 2011, 46(10): 4853-4858. [3]. Bu Y, Gao L, Smolinski M, et al. KXO1 (KX2-391), a Src-family kinase inhibitor targeting the peptide-binding domain, suppresses oncogenic proliferation in vitro and in vivo[J]. 2008. | |
| Cas No. | 897016-82-9 | SDF | |
| Synonyms | KX 01, Tirbanibulin | ||
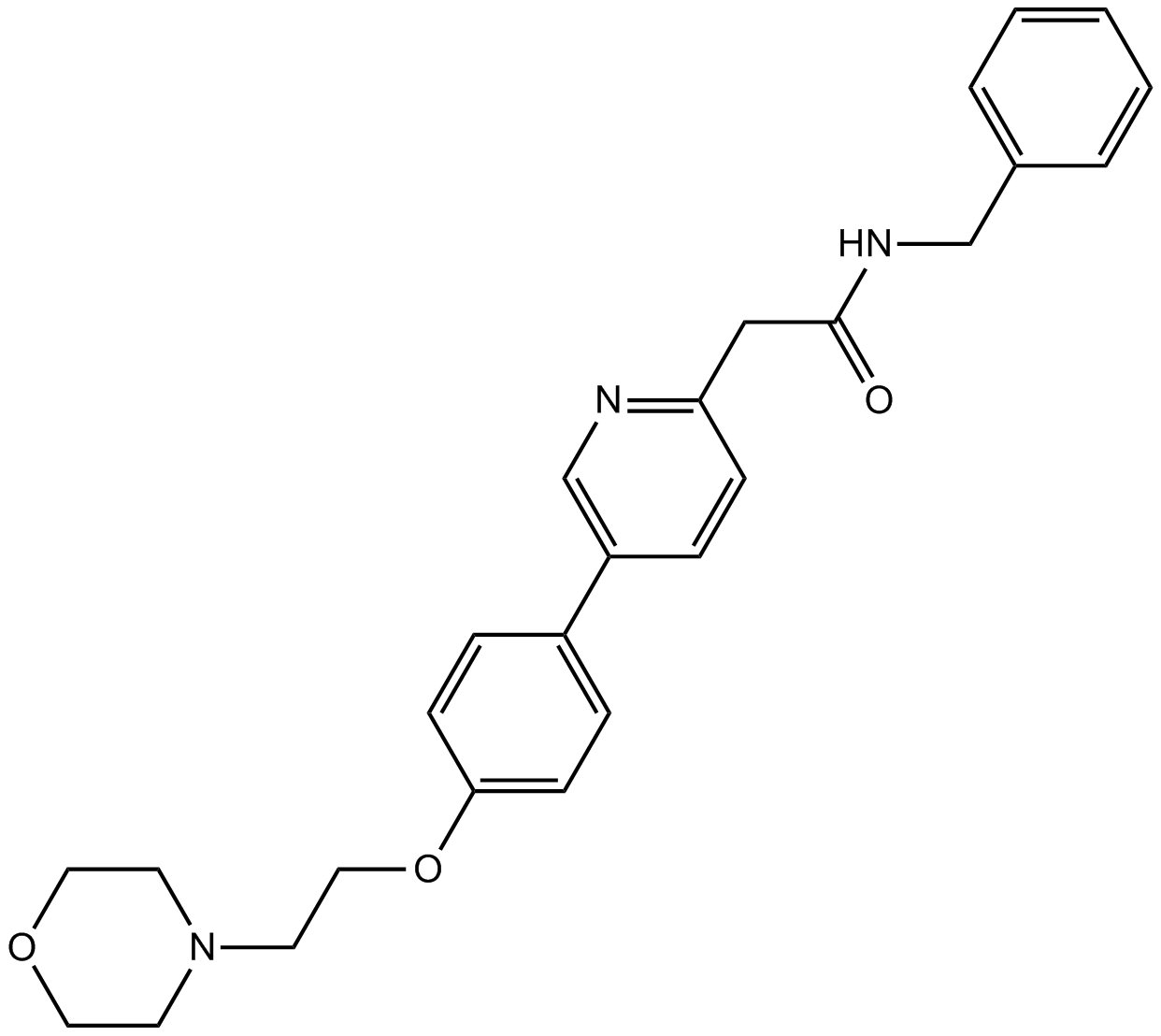
| Chemical Name | N-benzyl-2-[5-[4-(2-morpholin-4-ylethoxy)phenyl]pyridin-2-yl]acetamide | ||
| Canonical SMILES | C1COCCN1CCOC2=CC=C(C=C2)C3=CN=C(C=C3)CC(=O)NCC4=CC=CC=C4 | ||
| Formula | C26H29N3O3 | M.Wt | 431.53 |
| Solubility | ≥ 121mg/mL in DMSO | Storage | Store at -20°C |
| General tips | Please select the appropriate solvent to prepare the stock solution according to the
solubility of the product in different solvents; once the solution is prepared, please store it in
separate packages to avoid product failure caused by repeated freezing and thawing.Storage method
and period of the stock solution: When stored at -80°C, please use it within 6 months; when stored
at -20°C, please use it within 1 month. To increase solubility, heat the tube to 37°C and then oscillate in an ultrasonic bath for some time. |
||
| Shipping Condition | Evaluation sample solution: shipped with blue ice. All other sizes available: with RT, or with Blue Ice upon request. | ||
| Prepare stock solution | |||

|
1 mg | 5 mg | 10 mg |
| 1 mM | 2.3173 mL | 11.5867 mL | 23.1734 mL |
| 5 mM | 0.4635 mL | 2.3173 mL | 4.6347 mL |
| 10 mM | 0.2317 mL | 1.1587 mL | 2.3173 mL |
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
 g
g
 μL
μL

Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
3. All of the above co-solvents are available for purchase on the GlpBio website.
Quality Control & SDS
- View current batch:
- Purity: >99.00%
- COA (Certificate Of Analysis)
- SDS (Safety Data Sheet)
- Datasheet
Average Rating: 5 (Based on Reviews and 9 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *



















