Phomopsin A (Synonyms: NSC 381839) |
| Catalog No.GC15514 |
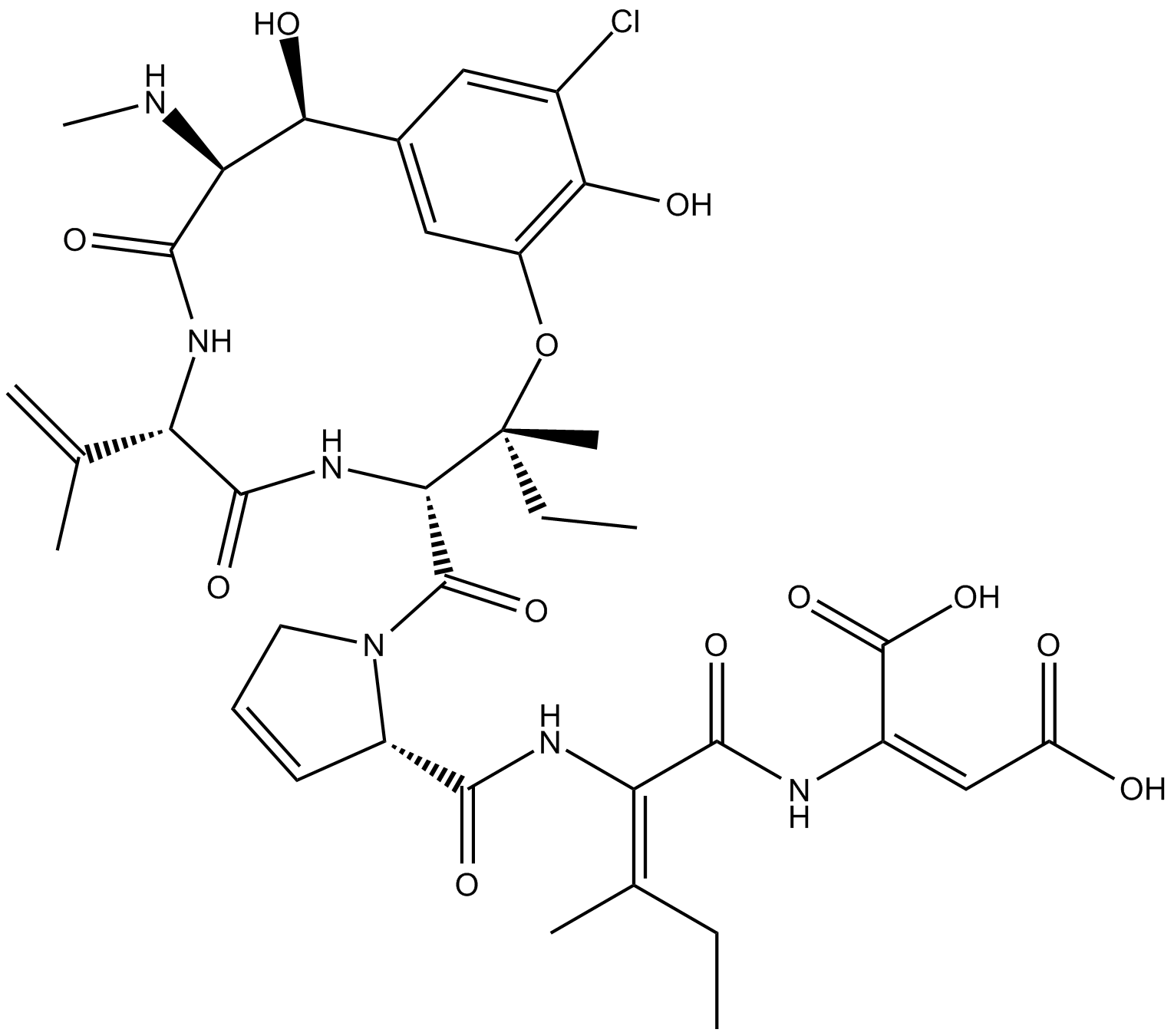
Phomopsin A는 Phomopsis leptostomiformis 곰팡이에서 분리된 환형 헥사펩티드 진균독소입니다. 포몹신 A는 방사성 표지된 빈크리스틴과 튜불린의 결합에 대한 비경쟁적 억제제입니다.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 64925-80-0
Sample solution is provided at 25 µL, 10mM.
Phomopsin A is a cyclic hexapeptide mycotoxin that inhibits β-tubulin.
Phomopsins are a family of mycotoxins produced by the fungus Phomopsis leptostomiformis grows on lupins, which cause lupinosis, a severe liver disease of grazing animals [1][2].
Microtubules are one of the major components of the cytoskeleton that are essential in several cellular functions such as cell division and morphogenesis. α- and β-tubulins polymerize into microtubules.
Phomopsin A is a cyclic hexapeptide mycotoxin that binds β-tubulin in a vinca domain, partly overlapping with the site targeted by vinblastine and other tubulin inhibitors [2][3]. Phomopsin A noncompetitively inhibited the binding of radiolabeled vinblastine to tubulin with IC50 and Ki values of 0.8 μM and 2.8 μM, respectively. Phomopsin A potently inhibited tubulin-dependent GTP hydrolysis and nucleotide exchange on tubulin [2]. Phomopsin A, a vinca domain antimitotic peptide, also inhibited microtubule assembly [3][4]. Phomopsin A inhibited microtubule growth, modulated the dynamics of microtubules, and induced the self-association of tubulin dimers into single-walled rings and spirals [4].
References:
[1]. Hamel E. Natural products which interact with tubulin in the vinca domain: maytansine, rhizoxin, phomopsin A, dolastatins 10 and 15 and halichondrin B. Pharmacol Ther. 1992;55(1):31-51.
[2]. Cormier A, Marchand M, Ravelli RB, et al. Structural insight into the inhibition of tubulin by vinca domain peptide ligands. EMBO Rep. 2008 Nov;9(11):1101-6.
[3]. Li Y, Kobayashi H, Hashimoto Y, et al. Binding selectivity of rhizoxin, phomopsin A, vinblastine, and ansamitocin P-3 to fungal tubulins: differential interactions of these antimitotic agents with brain and fungal tubulins. Biochem Biophys Res Commun. 1992 Sep 16;187(2):722-9.
[4]. Mitra A, Sept D. Localization of the antimitotic peptide and depsipeptide binding site on beta-tubulin. Biochemistry. 2004 Nov 9;43(44):13955-62.
Average Rating: 5 (Based on Reviews and 6 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















