Tideglusib (Synonyms: NP031112) |
| Catalog No.GC14465 |
비-ATP 경쟁적 GSK-3β 억제제
Products are for research use only. Not for human use. We do not sell to patients.
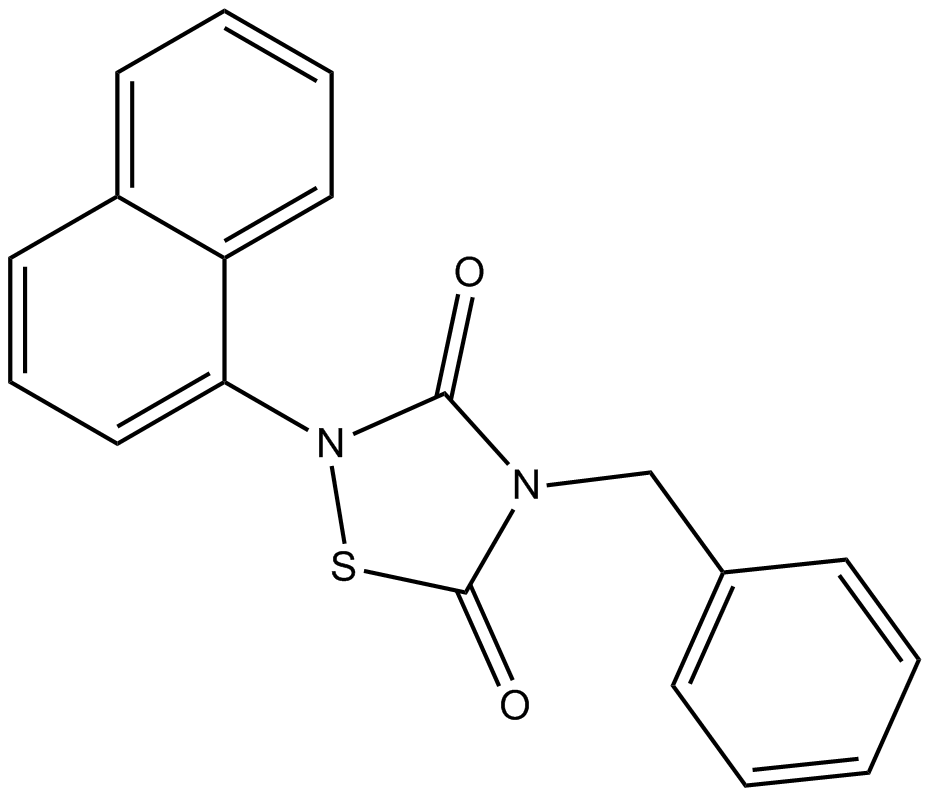
Cas No.: 865854-05-3
Sample solution is provided at 25 µL, 10mM.
IC50: A potent, selective and irreversible non-ATP-competitive GSK-3β suppressor with an IC50 of 60 nM.
Tideglusib is a GSK-3 inhibitor currently undergoing phase II clinical trials for Alzheimer disease and progressive supranuclear palsy. Sustained oral administration of Tideglusib to animal models could down-regulates Tau hyper-phosphorylation, reduces brain amyloid plaque load, promotes learning and memory as well as prevents neuronal loss. [1]
In vitro: In vitro studies showed that after the unbound Tideglusib was removed from the reaction medium, the enzyme function could not be recovered. In addition, the dissociation rate constant of the reaction was as low as nearly zero. All above findings suggested that Tideglusib blocked GSK-3 irreversibly. Such irreversibility might be responsible for the non-competitive inhibition pattern with respect to ATP of Tideglusib and perhaps other structurally related compounds. [1]
In vivo: Based on double transgenic mice model co-expressing human mutant APP and tau, a study demonstrated that Tideglusib could suppress GSK-3, reduced amyloid and tau pathologies, blocked neuronal cell death and memory deficits in vivo. [2]
Clinical trial: A pilot, double-blind, placebo-controlled and randomized clinical trial was conducted to study the effect of Tideglusib in AD patients with an escalating dose. Thirty patients with mild to moderate AD were orally administered with Tideglusib in escalating doses of 400, 600, 800 and 1000 mg for periods of 4, 4, 6 and 6 weeks, respectively. This pilot study proved the safety and effectiveness of Tideglusib in AD patients. [3]
References:
[1]Domínguez JM, Fuertes A, Orozco L, Monte-Millan MD, Delgado E and Medina M. Evidence for irreversible inhibition of glycogen synthase kinase-3 by Tideglusib. J Biol Chem. 2012 Jan; 287(2): 893-904.
[2]Serenóa L, Coma M, Rodríguez M, Sánchez-Ferrer P, Sánchez MB, Gich I, Agulló JM, Pérez M, Avila J, Guardia-Laguarta C, Clarimón J, Lleó A, Gómez-Isla T. A novel GSK-3β inhibitor reduces Alzheimer's pathology and rescues neuronal loss in vivo. Neurobiol Dis. 2009 Sep; 35(3): 359-67.
[3]del Ser, T. Phase IIA clinical trial on Alzheimer’s Disease with NP-12, a GSK-3 inhibitor. Alzheimers and Dement. 2010; 6: S147.
Average Rating: 5 (Based on Reviews and 8 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




