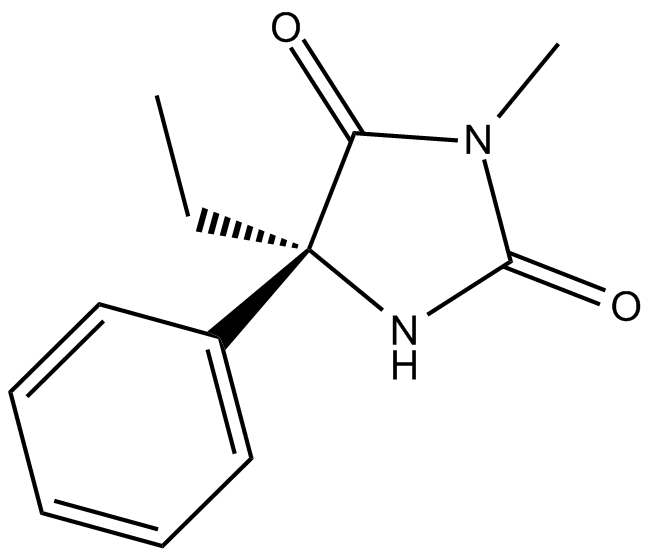
(S)-Mephenytoin (Synonyms: (S)-5-Ethyl-3-methyl-5-phenylhydantoin) |
| Catalog No.GC14486 |
S-Mephenytoin (5-phenyl-5-ethyl-N-methylhydantoin) is an anticonvulsive drug which is metabolized by N-demethylation and 4-hydroxylation (of the aromatic ring).
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 70989-04-7
Sample solution is provided at 25 µL, 10mM.
S-Mephenytoin (5-phenyl-5-ethyl-N-methylhydantoin) is an anticonvulsive drug which is metabolized by N-demethylation and 4-hydroxylation (of the aromatic ring). CYP2C19 genotype is a major determined factor for metabolisms of S-Mephenytoin. The formations of 4X-hydroxymephenytoin, S-Mephenytoin’s metabolite is CYP2C19 genotype dependent.[1][2]
The genetic polymorphism of CYP2C19 was revealed by the discovery of deficient 4’-hydroxylation of mephenytoin (3-methyl-5-phenyl-5 ethylhydantoin; Mesantoin). Human can be characterized as poor (PM) or extensive metabolizers (EM) with use of racemic mephenytoin as a phenotyping drug, and CYP2C19 has been identified as the major S-Mephenytoin (S-MP) hydroxylase in human. This polymorphism affects the metabolism of many other clinically important drugs such as diazepam, omeprazole (OP), imipramine, propranolol, and chloroguanide.[1]
Rabbit anti-human cytochrome b5 inhibited NADH-cytochrome-c reductase and S-Mephenytoin 4-hydroxylase activities in human liver microsomes. In the presence of cytochrome b5, the Km value for S-Mephenytoin was 1.25 mM with all five purified cytochrome P-450s preparations, and Vmax values were 0.8-1.25 nmol of 4-hydroxy product formed per min/nmol of P-450. P-45OMP is a relatively selective P-450 form that metabolizes substituted hydantoins well.[2]
References:
[1]. Zhou HH. CYP2C19 genotype determines enzyme activity and inducibility of S-Mephenytoin hydroxylase. Clin Chim Acta. 2001 Nov;313(1-2):203-8.
[2]. Shimada T, et al. Human liver microsomal cytochrome P-450 mephenytoin 4-hydroxylase, a prototype of genetic polymorphism in oxidative drug metabolism. Purification and characterization of two similar forms involved in the reaction. J Biol Chem. 1986 Jan 15;261(2):909-21.
Average Rating: 5 (Based on Reviews and 24 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




