3X Flag peptide
Flag peptide is the peptide sequence of amino acids, which is hydrophilic and has high immunogenicity. These are fusion peptides that are used as tags to detect the target molecule either protein or protein complexes for their rapid purification. It is consisting of almost 8 amino acids (DYKDDDDK) which are sequenced in a specific manner. These flag peptides are of small size; hence they are encoded by a short oligonucleotide sequence. Normally the last 5 sequences of amino acids serve as a specific cleavage site of an enzyme called enterokinase, that in turn allows the removal of the tag peptide. Depending upon the functions or applications of the flag peptide, it may either combine with the N or C terminal of the protein. The N-terminal of the given flag peptide has an affinity to bind with the monoclonal antibodies which are called anti-flag antibodies. The complex which is formed between the flag peptide and the monoclonal antibody is Ca2+ dependent. Which can be broken by any chelating agent like EDTA. Some studies contradict this concept by saying that the fusion between the flag peptide and the monoclonal antibodies M1 (Also called anti-flag antibodies) is not Ca2+ dependent.
3X flag peptide is a peptide sequence that is formed by the combination of 23 amino acids. It is hydrophilic in nature and can be used to detect fusion protein and metabolites which are bioactive in nature. It is also used along with immunoprecipitation (IP) to detect highly active metabolic products of the cell. Recently studies have been conducted along with the IP to determine the performance of 3X flag protein for the detection of target proteins for isolespedezate(Evans and Cravatt, 2006). The target protein which is detected using the 3X flag and IP is CMetE which acts as a molecular target for isolespedezate. This isolespedezate is present in the cassia plant and acts as a factor for leaf opening. The major thing is that the 3X flag peptide binds with this factor non-specifically. Studies also suggested that the use of a 3X Flag tag along with the combination of linkers is good enough to give us the best results for the purification of target proteins. But this success does not only depend upon the length of the linker but also depends upon its nature
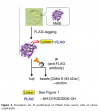
This 3X flag tag is a highly charged oligopeptide because it consists of 11 units of aspartate within the 23 sequences of amino acids. Due to this reason, it is highly important to improve the selectivity as well as the efficiency in the purification technique using IP. The major reason behind this is that it shows expand conformation, during an experiment in the aqueous solution because of the electrostatic repulsive forces, present between aspartate molecules. This tag peptide is a large charged molecule that has an affinity to bind with anti-flag antibodies. Figure-1 also describes the procedure by which the 3X flag peptide detects the target molecule. We have already discussed that the CMetE is the target molecule in the Cassia plant. Upon flag tagging, this target molecule combines with flag tag by a linker molecule. This complex upon IP, combine with the anti-flag antibodies, give bead.
3X flag peptide can also be used as a reagent for the purification of the recombinant proteins which are tagged by flag epitope. This 3X flag peptide has a strong affinity for the monoclonal antibodies ( anti-flag antibodies M1) in the presence of calcium, so in the absence of the Ca2+, these monoclonal 1 antibodies break the complex with flag-tagged peptides but on the other hand, still, these antibodies have an affinity for flag peptide even in the free metal state. Due to this reason, it is difficult to develop ELISA- assay which is metal sensitive. The reason behind it is that the antibodies have an affinity to bind with the coated antigen (have a polyvalent surface), for example when the flag peptide is in the ELISA plate, they bound to it because of the polyvalent nature of the antigen. However, when the antibodies are produced in monovalent nature by proteolytic cleavage then this process becomes reversed and the reaction will become Ca2+ dependent. Thus, the newly formed ELISA which is metal-dependent was helpful to determine the need for metal for antibodies and its complex formation with flag peptides. several metals which are smaller in size, they can act as an inhibitory moiety to stop the binding of the antibodies; for example, nickel. On the other side, for heavy metals like cadmium, there is a considerable binding ability for antibodies. This discovery of heavy metals for considerable binding is of great importance because these antibodies now can be used for the co-crystallization of recombinant flag-fusion proteins .
3X flag tag protein in the association of polyhistidine (His6) can also be used in tandem affinity purification. This method is of great importance because it is very helpful to detect those protein complexes that are associated with the gene of interest. They function in conjugation with the gene of interest, that’s why we can detect and purify out our target proteins or molecules. Previously many purification methods were used to purify target molecules, which include chromatography, affinity chromatography, gel filtration, etc. But these methods require sufficient purification levels to detect desirable protein complexes. But the recent techniques like affinity purification coupled with MS (Mass Spectrometry) are of great importance in which His6- 3X flag peptides are used to detect the target molecule(Huang and Nusinow, 2016).
PfuMBP (Pyrococcus furiosus maltodextrin binding protein) can form crystals, which can easily show diffraction upon X-ray contact. This is a model, which is used to investigate the effect of peptide tags on protein crystalization and X-ray diffraction ability. Studies suggest that the crystals which are formed in the presence of flag peptide and the crystals which are formed in the absence of tag peptide are similar and also their diffraction ability is the same. Hence these flag peptides do not have an influence on crystal formation.








Comentarios