RSM-932A
Introduction: RSM-932A, also known as TCD- 717, is a ChoKα asset, which targets the mortal choline kinase nascence, an enzyme involved in increased lipid metabolism of cancer cells. RSM-932A appears to have an altogether new medium of inhibition and is synergistic with respect to both choline and ATP. RSM-932A have in vitro inhibitory exertion in the low nanomolar range. RSM-932A significantly reduced parasitemia and convinced the accumulation of trophozoites and schizonts, blocking intraerythrocytic development and snooping with sponger exit or irruption, suggesting a detention of the sponger development stage.

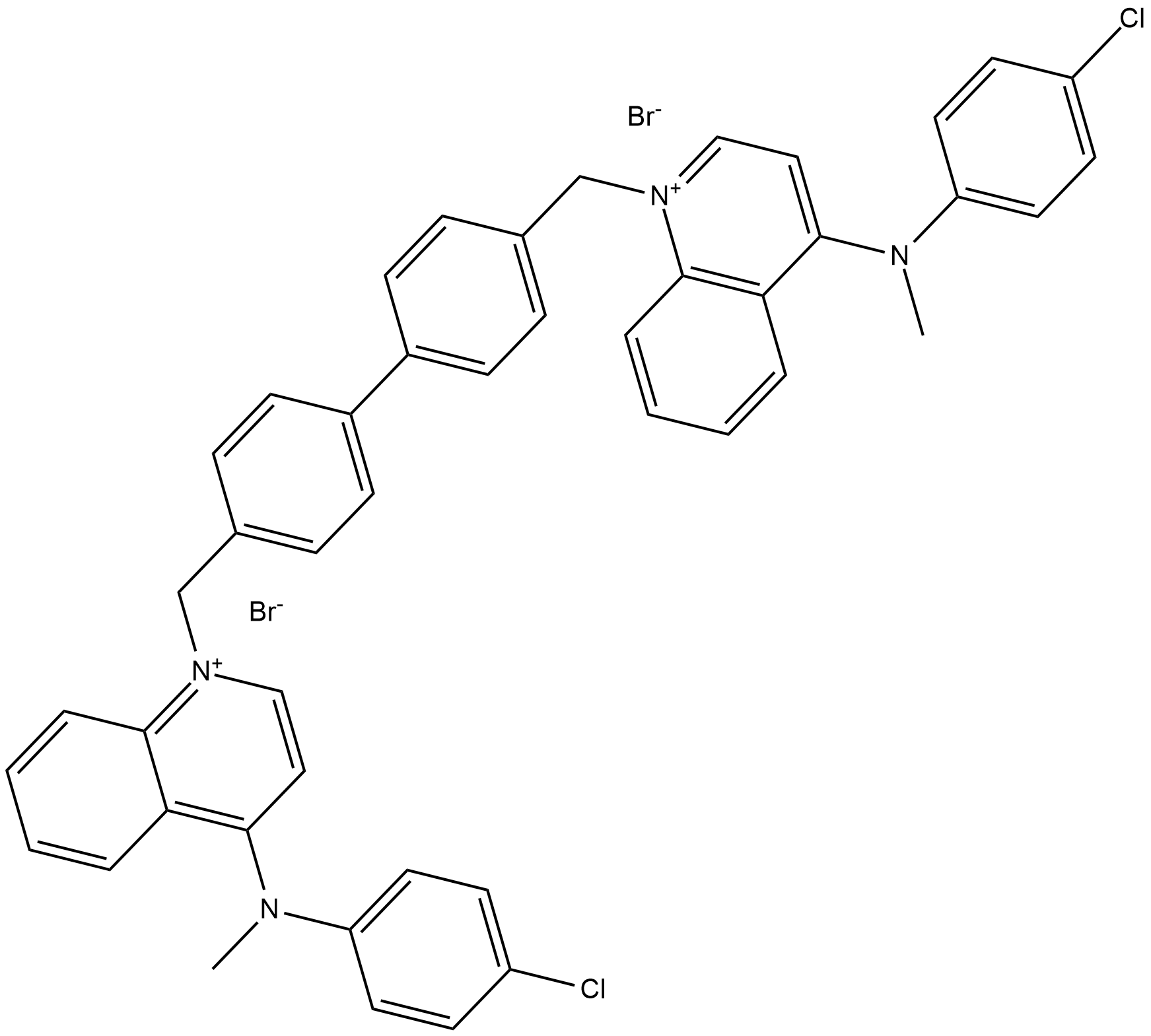
Figure 1: shows the chemical structure of RSM-932A.
Before we dwell into the mechanism of action of RSM-932A, it is necessary to understand the functions of ChoK and their disease relation.
What Choline Kinases are: Choline kinase (also known as CK, ChoK and choline phosphokinase) is an enzyme which catalyzes the first response in the choline pathway for phosphatidylcholine (PC) biosynthesis. This response involves the transfer of a phosphate group from adenosine triphosphate (ATP) to choline in order to form phosphocholine. ATP + choline ⇌ ADP + O-phosphocholine : Therefore, the two substrates of this enzyme are ATP and choline, whereas its two products are adenosine diphosphate (ADP) and O- phosphocholine. Choline kinase requires magnesium ions (2) as a cofactor for this response. This enzyme belongs to the family of transferases, specifically those transferring phosphorus- holding groups (phosphotransferases) with an alcohol group as acceptor. The first elaborate disquisition of the enzyme was conducted by McCamen in 1962, where it was shown that the brain is the richest source of the enzyme in mammalian towel. An affiliated enzyme, ethanolamine kinase, tends toco-purify with choline kinase leading to an indication that the two conditioning are intermediated by two distinct active spots on a single protein. The methodical name of this enzyme class is ATP:choline phosphotransferase. These enzymes share in glycine, serine and threonine metabolism and glycerophospholipid metabolism. In mammalian cells, the enzyme exists as three isoforms CKα- 1, CKα- 2 and CKβ. These isoforms are decoded by two different genes, CHKA and CHKB and are only active in their homodimeric, heterodimeric and oligomeric forms.
Oncogenic activity and CKα-1: Overexpression of CKα- 1 has been set up to be associated with cancer. Recent studies carried out on cancer cell lines have shown that CKα- 1 is over-expressed in bone cancer cells. This leads to an accumulation of phosphocholine in the bone and causes malice. Studies using colon, mortal lung and prostate lymphomas also revealed that CK is upregulated by overexpression of CKα- 1 in these cells compared to the normal, non-cancerous cells. One possible explanation for this is that CKα- 1 aids in the regulation of protein kinase B phosphorylation, particularly at the Serine- 473 end. Accordingly, high situations of expression and exertion of CKα- 1 promotes cell growth and survival. Grounded on the observation that increased exertion of CKα- 1 is related to cancer, CKα- 1 has promising use as a excrescence biomarker and in diagnosing and following the progression of excrescences. All mortal cancer cells have shown increased situations of this particular enzyme.
Muscular dystrophy and CKβ: It has been shown, using CKβ knockout mice models, that a disfigurement in the CKβ exertion leads to a drop in the phosphatidylcholine (PC) content in the hindlimb muscle. This, still, doesn't affect the phosphoethanolamine (PE) content. The net effect is also that the PC/ PE rate decreases and this leads to disabled membrane integrity in the liver. This compromised membrane eventuality leads to conking of the mitochondria. Although CK is needed for the biosynthesis of PC, CK is typically present in redundant and so isn't generally considered the rate- limiting step. Experimenters have concluded, still, that due to the reduced exertion of CK seen in the hindlimb muscle of the CKβ knockout mice model, CK is presumably the rate- limiting enzyme in cadaverous muscles. This suggests that disfigurement in CKβ may lead to a drop in PC conflation in the muscles performing in muscular dystrophy. These results suggest that CK could conceivably play a vital part in the homeostasis of PC.
Lipid metabolic reprogramming in cancer: Excrescence cells enhance lipid metabolism by adding exogenous lipid uptake and lipid conflation, leading to increased intracellular lipid content. Upregulation of lipid transport proteins similar as CD36, FATPs, FABPs, and LDLR increases lipid uptake. These upregulations increase intracellular SUFA, PUFA, and cholesterol situations. Meanwhile, endogenous lipid conflation originates from citrate in the TCA cycle, as well as intracellular glutamine, lactate and acetate, leading to the conflation of SFA and cholesterol. The process is catalyzed by crucial enzymes similar as FASN and SCD. FAs in excrescence cells are catalyzed by ACSL to form acyl- CoA, which is involved in the posterior conflation of intracellular phospholipids with bioactive lipids and FAO. Acyl- CoA facilitates the translocation of enzymes into mitochondria through CPT1, the crucial enzyme of FAO, sharing in the product of acetyl- CoA, furnishing energy for the natural gets of excrescence cells. redundant lipids in excrescence cells are stored in LD as CE and Label. This storehouse significantly prevents LPO and attenuates its threat of interceding excrescence cell death.

Figure 2: shows the lipid metabolic reprogramming in cancer cells.
Preclinical Experiment on Mice Regarding ChoK Inhibitors: The experimental research on mice regarding RSM-932A has been focused on its preclinical characterization as a novel anticancer drug targeting human choline kinase alpha (ChoKα). Here’s a summary of the key findings: ChoKα Impediments A set of ChoKα impediments, including RSM-932A, was generated with potent antiproliferative exertion in vitro against colorful excrescence- deduced cell lines. In Vivo Antitumoral Activity These impediments demonstrated significant antitumoral exertion in vivo against mortal xenografts in mice, indicating high efficacity. Low toxin Biographies The composites showed low toxin biographies at effective boluses, which is pivotal for implicit remedial operations. Clinical Development Among the impediments, RSM-932A was chosen for farther clinical development due to its strong in vitro and in vivo anticancer exertion and lack of toxin at effective boluses. Support for Clinical Trials The preclinical substantiation supports the use of RSM-932A as a seeker for clinical trials as the “first in humans” medicine targeting ChoKα. The research indicates that RSM-932A has the potential to be an effective anticancer therapy with a novel mechanism of action targeting the lipid metabolism of cancer cells. The promising results from the preclinical studies in mice provide a strong foundation for advancing RSM-932A into clinical trials.

Figure 3: shows the Efficacy of selected ChoKα inhibitors against the human colon cancer cell line HT-29 xenograft .
Mechanism of action of RSM-9322A: The mechanism of action of RSM-932A involves the inhibition of ChoKα, leading to a disruption in the synthesis of phosphatidylcholine (PC), a major component of cell membranes. Here’s a simplified explanation of its mechanism: Inhibition of ChoKα: RSM-932A binds to ChoKα and inhibits its activity. ChoKα is responsible for the phosphorylation of choline to form phosphocholine (PCho), which is a key step in the CDP-choline pathway for synthesizing PC. Disruption of Lipid Metabolism: By inhibiting ChoKα, RSM-932A decreases the levels of PCho and disrupts the lipid metabolic pathways that are essential for the proliferation and survival of tumor cells. Antiproliferative Effect: The disruption of lipid metabolism leads to an antiproliferative effect on cancer cells, as they rely on these pathways for growth and maintenance of their cell membrane structure. Synergistic Action: RSM-932A has a novel mechanism of inhibition that is synergistic with respect to both choline and ATP, meaning it can enhance the effectiveness of other compounds that target these molecules.
RSM-932A has shown potent antiproliferative activity in vitro against various tumor-derived cell lines and significant in vivo anticancer activity in human xenografts in mice, with a high efficacy and low toxicity profile. This makes it a promising candidate for further clinical development and potential use in cancer treatment.










Comentarios