Sodium Palmitate
The sodium salt of palmitic acid, sodium palmitate ( Hexadecanoic acid sodium salt, Palmitic acid sodium salt) , produced during the process of fatty acid synthesis present in fats and waxes. It is produced synthetically and driven by palm oil. Plays major role as an emulsifier. Emulsification keeps the tendency to separate two immiscible ingredients from mixing with each other. The soap is created by the saponification of oils and fats. The presence of sodium palmitate in the soap basically indicates the purpose of surfactant. The deep cleaning of skin and clothes is done by the breakdown of surface tension between two liquids that is done by surfactants.
Figure 1 : shows the chemical structure of sodium palmitate.
A short term (90-min) exposure to palmitate moderately increased JC-1 J-aggregate fluorescence (Fig. 1), which gives a quantitative estimation. The observation of the effect was done at 0.1mm sodium palmitate and reach the maximum concentration of 0.2mm of sodium palmitate. A rapid and pronounced loss of JC-1 fluorescence (Fig. 2) is done by the uncoupler FCCP. The FCCP effect was maximum at1 g/mol and only partial (20%) at 0.1 g/ml (Fig. 3). Culture for 24 h in the presence of 0.2mm sodium palmitate induced a 24% decrease in JC-1 fluorescence in the presence of 28 mm glucose (Fig. 4). At 2.8 mm glucose, however, there was only a non-significant trend for lower JC-1 fluorescence in response to sodium palmitate.

Figure 2. Short term effects of palmitate and FCCP on JC-1 fluorescence in dispersed islet cells. Isolated rat islets were loaded with 10 g/ml JC-1 for 20 min at room temperature. The islets were dispersed, and cells were placed in 96-well plates. At time zero, basal fluorescence was determined, and additions of palmitate and FCCP (1 g/ml) were made. Fluorescence was followed for 90 min, and the results are expressed as the change in fluorescence(arbitrary units)compared with that at time zero. Results are means of three observations.

Figure. 3. Short term effects of FCCP on JC-1 fluorescence in dispersed islet cells. Isolated rat islets were loaded with 10 g/ml JC-1 for 20 min at room temperature. The islets were dispersed, and cells were placed in 96-well plates. At time zero, basal fluorescence was determined, and additions of different concentrations of FCCP were made. Fluorescence was determined after 90 min, and the results are expressed as the change in fluorescence (arbitrary units) compared with that at time zero. Results are the mean SEM for three separate experiments.
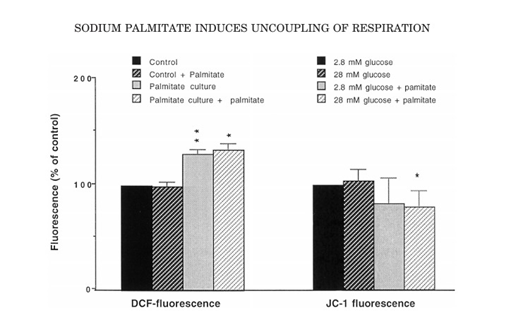
Figure. 4. Long-termeffectsofpalmitateonDCFHandJC-1fluorescence.
The effect of free fatty acids of sodium palmitate on the in-vitro culturing of human pancreatic islets is death. This observation is in contrast to Endo C-BH1 cells which do not die in response to sodium palmitate. The Endo C-BH1 cells show characteristic similarity with human beta cells but when it comes to free fatty acids e.g sodium palmitate induced lipotocxicity there is no similarity between Endo C-BH1 cells and human beta cells. It can be hypothesized that a different culture condition may render also EndoC-βH1 cells sensitive to sodium palmitate. Sodium palmitate was dissolved in 50% ethanol during heating to 60°C (final concentration of ethanol: 0.50%) and was added to the 2% fatty acid free BSA (Roche) containing culture media 30 min before addition to the cell cultures.
A paired comparison between culture in DMEM with DMEM/F12 demonstrated a markedly increased sensitivity of EndoC-βH1 cells to the apoptotic effects sodium palmitate and sodium palmitate + high glucose (Fig. 4). Time course analysis demonstrated that already after a one day DMEM/F12 culture period palmitate + high glucose increased cell death markedly, and at day 2 and 3 also palmitate alone, at 5.5 mM glucose, promoted increased cell death (Fig. 4A). Using DMEM, however, palmitate + high glucose induced only a small increase in cell death at day 3. High glucose by itself did not affect cell death rates; neither in DMEM nor DMEM/F12 cultured cells. The dose response, analyzed after a 24 h palmitate exposure period, revealed that a concentration of 1.5 mM palmitate in DMEM/F12 medium is sufficient to promote substantial EndoC-βH1 cell death, and that 22 mM of glucose potentiates the effect of palmitate (Fig. 4B).
As DMEM/F12 contains linoleic acid, whereas DMEM does not, we next analyzed whether supplementation of DMEM with linoleic acid (84 μg/L) mimicked the effect of DMEM/F12. Indeed, linoleic acid promoted a modest increase in cell death when co-cultured in palmitate + high glucose containing DMEM (Fig. 4C). We also analyzed cell death in response to the cytokines interleukin-1β + IFN-γ, and in this case DMEM/F12 did not potentiate cell death rates as compared with DMEM (Fig. 4D).


Figure 4. EndoC-βH1 cells are sensitive to sodium palmitate when cultured in DMEM/F12. Human EndoC-βH1 cells were pre-cultured in ECM/fibronectin-coated plates in 5.5 mM glucose Palmitate was dissolved in 50 % ethanol during heating to 60°C and added to the culture medium containing 2% albumin (final concentration of ethanol: 0.5 %). At control conditions (C), the glucose concentration was 5.5 mM, and at high glucose conditions (HG), the glucose concentration was 22 mM. In the time dependency analysis (A), the palmitate concentration was 1.5 mM. In the dose response analysis (B) the palmitate concentrations were 1.0, 1.5 and 2.0 mM, and the time point was 24 h. Cell death was determined using propidium iodide staining and flow cytometry. Results are means ± SEM for 4 independent experiments. *denotes p < 0.05 using one-way ANOVA and Fisher's LPD post-hoc test. In (C), cells were cultured for 2 d with or without 84 μg/L linoleic acid, palmitate (P, 1.5 mM) and high glucose (HG, 22 mM) as indicated in the Figure. Results are means ± SEM for 4 independent experiments. *and** denote p < 0.05 and p < 0.01, respectively, using Student's paired t-test. In (D) EndoC-βH1 cells were cultured for 2 d in DMEM or DMEM/F12 as described above with our without IL-1β (10 ng/ml) + IFN-γ (10 ng/ml). Results are means ± SEM for 5 independent experiments.
FFAR1 and FFAR4 are critical lipid metabolism regulators and trigger PPARγ activation. As shown in Fig. 5, GSK and GW+GSK improved the expression of PPARγ. The utilization of FFAs with an OA:PA of 2:1 to induce lipid accumulation in RAW264.7 cells, and the effects of GW9508 and GSK137647 on fatty acid metabolism were observed.

Fig. 5. FFAR1 and FFAR4 agonists activated PPARγ and regulated fatty acid metabolism based on DSS-induced colitis mice. The mRNA level of PPARγ (A), ACC1 (B), FASN (C), SCD1 (D), and CPT-1α (E). Data are presented as mean ± SD (n = 6).
In short , sodium palmitate is abundant in the human body and plays a significant role in various cellular processes, including protein modifications and inflammation regulation. Sodium palmate is also found in cleansing products such as soaps and body washes. Sodium palmate for face and body acts as a surfactant in soaps and body washes to remove dirt and oil from the skin.













Comentarios