c-di-AMP |
| Catalog No.GC13643 |
El c-di-AMP (diadenilato cÍclico) es un agonista de STING, que se une a la proteÍna transmembrana STING, activando asÍ la vÍa de seÑalizaciÓn TBK3-IRF3 y, posteriormente, desencadenando la producciÓn de IFN tipo I y TNF.
Products are for research use only. Not for human use. We do not sell to patients.
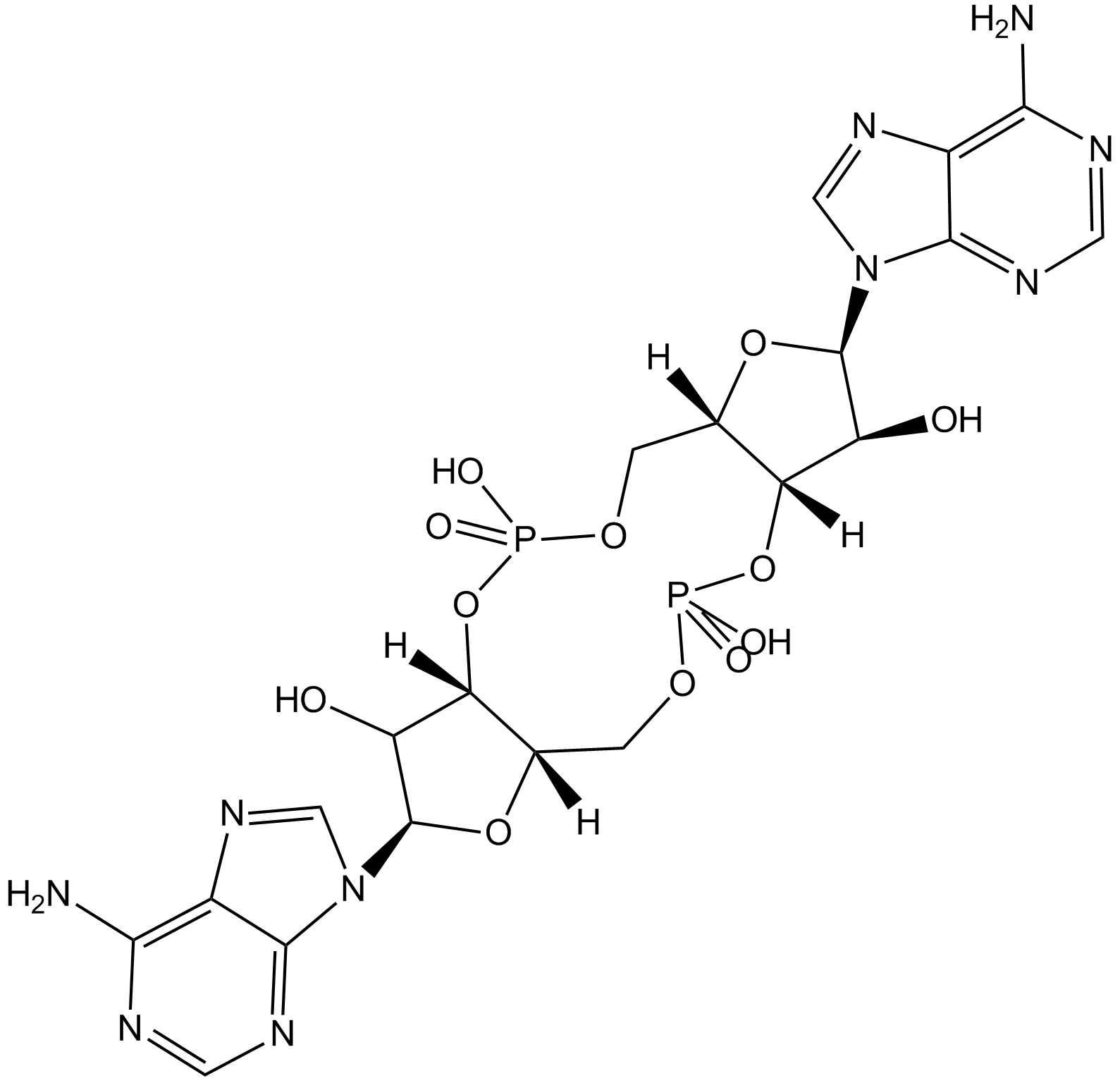
Cas No.: 54447-84-6
Sample solution is provided at 25 µL, 10mM.
C-di-AMP is a STING agonist, which binds to the transmembrane protein STING, thereby activating the TBK3-IRF3 signaling pathway, subsequently triggering the production of type I IFN and TNF. c-di-AMP serves as a second messenger in bacteria, primarily regulating cell growth, survival, and virulence in Gram-positive bacteria, and also modulates the host immune response. As an effective mucosal adjuvant, c-di-AMP stimulates both humoral and cellular responses[1-5]. C-di-AMP have been utilized as vaccine adjuvants for influenza and hepatitis C.Citation[6].
Combination of c-di-AMP(5 ug/ml;48h) with radiation promotes the secretion of type I IFN and ISG levels in BMDCs[7].
C-di-AMP(25ug;i.p;3 times) can synergistically enhance the antitumor effects of Radiotherapy (RT)-associated dsDNA by promoting IFN-β production and CD8+ cytolytic T cell activation in a cGAS- and STING-dependent manner in mice[7].
References:
[1]. Jenal U, Reinders A, et,al.Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol. 2017 May;15(5):271-284. doi: 10.1038/nrmicro.2016.190. Epub 2017 Feb 6. PMID: 28163311.
[2]. Fahmi T, Port GC, et,al.c-di-AMP: An Essential Molecule in the Signaling Pathways that Regulate the Viability and Virulence of Gram-Positive Bacteria. Genes (Basel). 2017 Aug 7;8(8):197. doi: 10.3390/genes8080197. PMID: 28783096; PMCID: PMC5575661.
[3]. Ning H, Wang L, et,al. Recombinant BCG With Bacterial Signaling Molecule Cyclic di-AMP as Endogenous Adjuvant Induces Elevated Immune Responses After Mycobacterium tuberculosis Infection. Front Immunol. 2019 Jul 3;10:1519. doi: 10.3389/fimmu.2019.01519. PMID: 31333655; PMCID: PMC6618344.
[4]. Ebensen T, Delandre S, et,al. The Combination Vaccine Adjuvant System Alum/c-di-AMP Results in Quantitative and Qualitative Enhanced Immune Responses Post Immunization. Front Cell Infect Microbiol. 2019 Feb 19;9:31. doi: 10.3389/fcimb.2019.00031. PMID: 30838180; PMCID: PMC6390046.
[5]. Sanchez MV, Ebensen T, et,al. Intranasal delivery of influenza rNP adjuvanted with c-di-AMP induces strong humoral and cellular immune responses and provides protection against virus challenge. PLoS One. 2014 Aug 20;9(8):e104824. doi: 10.1371/journal.pone.0104824. PMID: 25140692; PMCID: PMC4139298.
[6]. Yin W, Cai X, et,al. A decade of research on the second messenger c-di-AMP. FEMS Microbiol Rev. 2020 Nov 24;44(6):701-724. doi: 10.1093/femsre/fuaa019. PMID: 32472931; PMCID: PMC7850090.
[7]. Li Z, Zhang Y, et,al. Gut microbiota modulate radiotherapy-associated antitumor immune responses against hepatocellular carcinoma Via STING signaling. Gut Microbes. 2022 Jan-Dec;14(1):2119055. doi: 10.1080/19490976.2022.2119055. PMID: 36093568; PMCID: PMC9467592.
Average Rating: 5 (Based on Reviews and 33 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















