Citric acid |
| Catalog No.GC14203 |
El Ácido cÍtrico es un conservante natural y potenciador de la acidez de los alimentos.
Products are for research use only. Not for human use. We do not sell to patients.
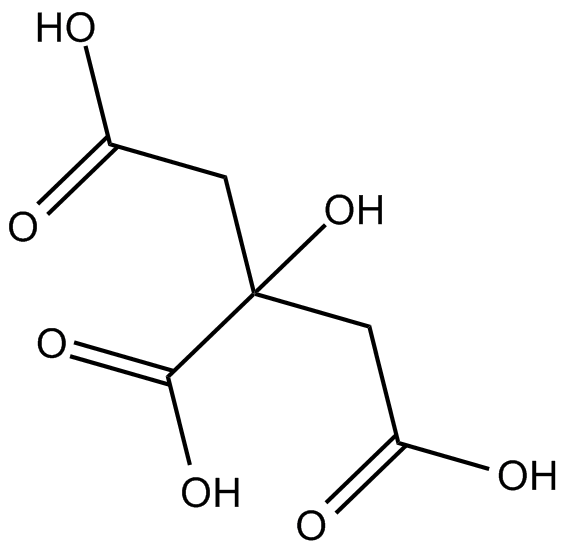
Cas No.: 77-92-9
Sample solution is provided at 25 µL, 10mM.
Citric acid is a weak organic tricarboxylic acid found in citrus fruits. Citric acid is a natural preservative and food tartness enhancer.
Citric acid induces apoptosis through the mitochondrial pathway in the human keratinocyte cell line HaCaT. It inhibits proliferation of HaCaT cells in a dose-dependent manner, but also induces apoptosis and cell cycle-arrest at the G2/M phase (before 24 h) and S phase (after 24 h)[1].
Citric acid is found in all animal tissues as an intermediary substance in oxidative metabolism. The administration of citric acid (1–2 g/kg) attenuates LPS-induced elevations in brain MDA, nitrite, TNF-α, GPx, and PON1 activity. In the liver, nitrite is decreased by 1 g/kg citric acid. Citric acid (1-2 g/kg) decreases brain lipid peroxidation and inflammation, liver damage, and DNA fragmentation[2]. Citric acid supplementation increases intestinal calcium and phosphorus absorption and the retention/intake ratio only in rats fed the 1% Ca diet. Citric acidsupplementation together with a calcium-rich diet allows to obtain an increased retention of calcium and phosphorus in bone. The prolonged administration of calcium citrate supplements may therefore help to increase bone mineral concentration[3]. Oral administration of citric acid ameliorates ketosis and protects against the development of diabetic complications in an animal model of type 1 diabetes[4].
Reference:
[1]. Ying TH, et al. Citric acid induces cell-cycle arrest and apoptosis of human immortalized keratinocyte cell line (HaCaT) via caspase- and mitochondrial-dependent signaling pathways. Anticancer Res. 2013 Oct;33(10):4411-20.
[2]. Abdel-Salam OM, et al. Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice. J Med Food. 2014 May;17(5):588-98.
[3]. Lacour B, et al. Stimulation by citric acid of calcium and phosphorus bioavailability in rats fed a calcium-rich diet. Miner Electrolyte Metab. 1997;23(2):79-87.
[4]. Nagai R, et al. Citric acid inhibits development of cataracts, proteinuria and ketosis in streptozotocin (type 1) diabetic rats. Biochem Biophys Res Commun. 2010 Feb 26;393(1):118-22.
|
Cell experiment: |
HaCaT cells are treated with different concentrations of citric acid (2.5, 5, 7.5, 10, 12.5 mM) for 24 h; 0.5% of DMSO (vehicle) is used as a control. Cells are then centrifuged at 1000 ×g for 5 min, and cell pellets are dissolved with 0.5 mL of Phosphate buffered saline (PBS) containing 5 μg/mL PI and viable cells are determined by using a flow cytometer for determination of viable cells[1]. |
|
Animal experiment: |
Rats: Rats are induced of diabetes and divided randomly into an untreated diabetic group and two treated diabetic groups, receiving citric acid (2 g/L) in the drinking water, or insulin therapy. Insulin-treated rats receive 3 IU of neutral protamine Hagedorn insulin three times per week. Fasting (12 hr) blood samples are obtained from the tail vein two days after insulin injection for measurement of blood glucose, HbA1c and ketone bodies[4]. [2]Mice: Citric acid is prepared in sterile physiological saline. Mice are randomly divided into five equal groups (six mice each). Mice are treated with either 0.2mL of: sterile physiological saline (group 1) or citric acid at doses of 1, 2, and 4 g/kg, orally (groups 2-4). Treatments are given just prior to endotoxin administration (LPS: 200 lg/kg, injected intraperitoneally, 0.1 mL). The fifth group received just the vehicle, no LPS (negative control). Mice are euthanized after 4 h of LPS or vehicle injection by decapitation under ether anesthesia, where the brain and liver of each mouse are then removed for analysis[2]. |
|
References: [1]. Ying TH, et al. Citric acid induces cell-cycle arrest and apoptosis of human immortalized keratinocyte cell line (HaCaT) via caspase- and mitochondrial-dependent signaling pathways. Anticancer Res. 2013 Oct;33(10):4411-20. |
|
| Cas No. | 77-92-9 | SDF | |
| Chemical Name | 2-hydroxypropane-1,2,3-tricarboxylic acid | ||
| Canonical SMILES | OC(CC(O)=O)(C(O)=O)CC(O)=O | ||
| Formula | C6H8O7 | M.Wt | 192.12 |
| Solubility | ≥ 19.2mg/mL in Water | Storage | Store at RT |
| General tips | Please select the appropriate solvent to prepare the stock solution according to the
solubility of the product in different solvents; once the solution is prepared, please store it in
separate packages to avoid product failure caused by repeated freezing and thawing.Storage method
and period of the stock solution: When stored at -80°C, please use it within 6 months; when stored
at -20°C, please use it within 1 month. To increase solubility, heat the tube to 37°C and then oscillate in an ultrasonic bath for some time. |
||
| Shipping Condition | Evaluation sample solution: shipped with blue ice. All other sizes available: with RT, or with Blue Ice upon request. | ||
| Prepare stock solution | |||

|
1 mg | 5 mg | 10 mg |
| 1 mM | 5.2051 mL | 26.0254 mL | 52.0508 mL |
| 5 mM | 1.041 mL | 5.2051 mL | 10.4102 mL |
| 10 mM | 0.5205 mL | 2.6025 mL | 5.2051 mL |
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
 g
g
 μL
μL

Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
3. All of the above co-solvents are available for purchase on the GlpBio website.
Quality Control & SDS
- View current batch:
- Purity: >98.00%
- COA (Certificate Of Analysis)
- SDS (Safety Data Sheet)
- Datasheet
Average Rating: 5 (Based on Reviews and 9 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *



















