Cytochalasin D (Synonyms: NSC 209835) |
| Catalog No.GC13440 |
La citocalasina D (Zygosporin A) es un potente inhibidor de la polimerizaciÓn de actina, podrÍa derivarse de un hongo.
Products are for research use only. Not for human use. We do not sell to patients.
Cas No.: 22144-77-0
Sample solution is provided at 25 µL, 10mM.
The cytochalasins are cell-permeable fungal metabolites that inhibit actin polymerization.[1],[2],[3],[4] This interferes with such diverse processes as cell movement, growth, phagocytosis, degranulation, and secretion.[5],[6],[7],[8] Cytochalasin D is a cell-permeable inhibitor that binds actin filaments, but not actin monomers, to inhibit polymerization at concentrations as low as 0.2 µM.2 In this way, it prevents the migration of tumor cells.[9]
Reference:
[1]. Brenner, S.L., and Korn, E.D. The effects of cytochalasins on actin polymerization and actin ATPase provide insights into the mechanism of polymerization. The Journal of Biological Chemisty 255(3), 841-844 (1980).
[2]. Lin, D.C., Tobin, K.D., Grumet, M., et al. Cytochalasins inhibit nuclei-induced actin polymerization by blocking filament elongation. Journal of Cell Biology 84, 455-460 (1980).
[3]. Ostlund, R.E., Jr., Leung, J.T., and Hajek, S.V. Regulation of microtubule assembly in cultured fibroblasts. Journal of Cell Biology 85, 386-391 (1980).
[4]. Pinder, J.C., and Gratzer, W.B. Structural and dynamic states of actin in the erythrocyte. Journal of Cell Biology 96(3), 768-775 (1983).
[5]. Flaumenhaft, R., Dilks, J.R., Rozenvayn, N., et al. The actin cytoskeleton differentially regulates platelet α-granule and dense-granule secretion. Blood 105(10), 3879-3887 (2005).
[6]. Taheri-Talesh, N., Horio, T., Araujo-Bazán, L., et al. The tip growth apparatus of Aspergillus nidulans. Molecular Biology of the Cell 19, 1439-1449 (2008).
[7]. dos Santos, T., Varela, J., Lynch, I., et al. Effects of transport inhibitors on the cellular uptake of carboxylated polystyrene nanoparticles in different cell lines. PLoS One 6(9), 1-10 (2011).
[8]. Nightingale, T.D., White, I.J., Doyle, E.L., et al. Actomyosin II contractility expels von Willebrand factor from Weibel-Palade bodies during exocytosis. Journal of Cell Biology 194(4), 613-629 (2011).
[9]. Hayot, C., Debeir, O., Van Ham, P., et al. Characterization of the activities of actin-affecting drugs on tumor cell migration. Toxicology and Applied Pharmacology 211, 30-40 (2006).
| Cell experiment [1-3]: | |
|
Cell lines |
HeLa, Vero, L, HEp2, and MDBK cells, SC-1 cells, Murine CT26 colorectal carcinoma cells |
|
Preparation method |
The solubility of this compound in DMSO is > 10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
|
Reacting condition |
0.2–0.5 μg/ml |
|
Applications |
In HeLa, Vero, L, HEp2, and MDBK cells, cytochalasin D (0.2–0.5 μg/ml) induced sustained contraction (contracture), loss of microvilli, expression of endoplasmic contents (zeiosis), nuclear protrusion, and extension of cytoplasmic processes. Cells in G1 were most sensitive to CD; responsiveness decreased progressively during early S and is least in mid S through G2. CD inhibited transport of [14C]deoxyglucose in HeLa. In SC-1 cells, Cytochalasin D treatment severely disrupted network organization, increased the number of actin filament ends, and led to the formation of filamentous aggregates or foci composed mainly of actin filaments. Cytochalasin D (0.24~15 μg/mL, 16 h) inhibited CT26 tumor cell proliferation in time and dose dependent manner and induced significant CT26 cell apoptosis. |
| Animal experiment [3,4]: | |
|
Animal models |
Murine CT26 tumor model, porcine coronary model |
|
Dosage form |
Intravenous injection, 50 mg/kg, every 3 days for 21 days |
|
Application |
Cytochalasin D (i.v., 50 mg/kg) in vivo treatment significantly inhibited tumor growth and prolonged the survival times in CT26 tumor-bearing mice. In porcine coronary model, Cytochalasin D (2 μg) resulted in less late lumen loss in low-dose. High-dose Cytochalasin D (20 μg) inhibited both late lumen loss and intimal area. |
|
Other notes |
Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
|
References: [1]. Miranda A F, Godman G C, Deitch A D, et al. Action of cytochalasin D on cells of established lines[J]. The Journal of cell biology, 1974, 61(2): 481-500. [2]. Schliwa M. Action of cytochalasin D on cytoskeletal networks[J]. The Journal of cell biology, 1982, 92(1): 79-91. [3]. Huang F Y, Li Y N, Mei W L, et al. Cytochalasin D, a tropical fungal metabolite, inhibits CT26 tumor growth and angiogenesis[J]. Asian Pacific journal of tropical medicine, 2012, 5(3): 169-174. [4].Salu K J, Bosmans J M, Huang Y, et al. Effects of cytochalasin D-eluting stents on intimal hyperplasia in a porcine coronary artery model[J]. Cardiovascular research, 2006, 69(2): 536-544. |
|
| Cas No. | 22144-77-0 | SDF | |
| Sinónimos | NSC 209835 | ||
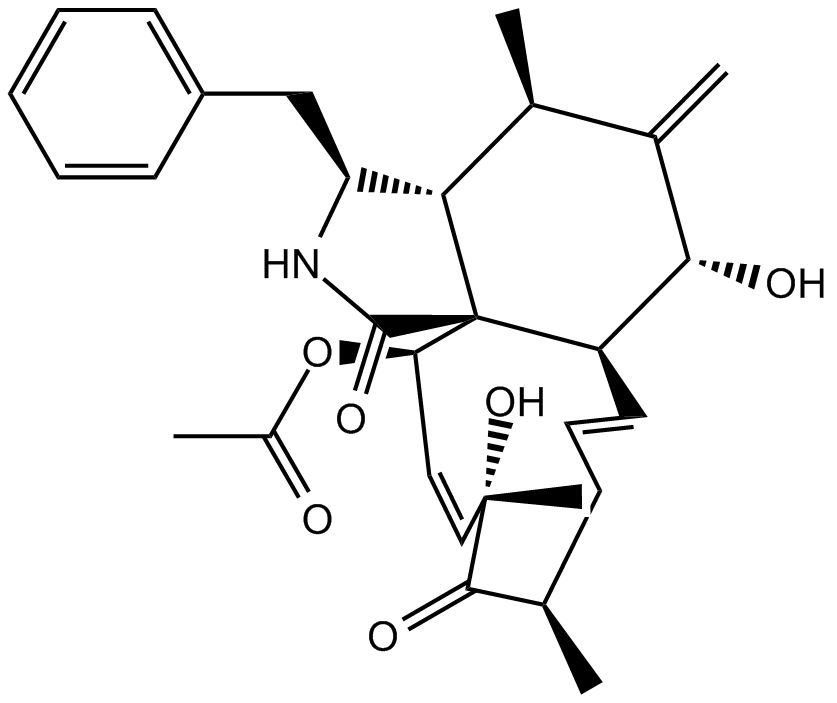
| Chemical Name | (3S,3aR,4R,6R,6aS,7E,10R,12S,13Z,15R,15aS)-3-benzyl-6,12-dihydroxy-4,10,12-trimethyl-5-methylene-1,11-dioxo-2,3,3a,4,5,6,6a,9,10,11,12,15-dodecahydro-1H-cycloundeca[d]isoindol-15-yl acetate | ||
| Canonical SMILES | O[C@@H]1[C@@H](/C=C/C[C@@H](C)C2=O)[C@]3([C@@H](C=C[C@]2(C)O)OC(C)=O)[C@@H]([C@@H](C)C1=C)[C@H](CC4=CC=CC=C4)NC3=O | ||
| Formula | C30H37NO6 | M.Wt | 507.63 |
| Solubility | 10mg/mL in dichloromethane,100 mg/ml in DMSO | Storage | Store at -20°C, protect from light |
| General tips | Please select the appropriate solvent to prepare the stock solution according to the
solubility of the product in different solvents; once the solution is prepared, please store it in
separate packages to avoid product failure caused by repeated freezing and thawing.Storage method
and period of the stock solution: When stored at -80°C, please use it within 6 months; when stored
at -20°C, please use it within 1 month. To increase solubility, heat the tube to 37°C and then oscillate in an ultrasonic bath for some time. |
||
| Shipping Condition | Evaluation sample solution: shipped with blue ice. All other sizes available: with RT, or with Blue Ice upon request. | ||
| Prepare stock solution | |||

|
1 mg | 5 mg | 10 mg |
| 1 mM | 1.9699 mL | 9.8497 mL | 19.6994 mL |
| 5 mM | 0.394 mL | 1.9699 mL | 3.9399 mL |
| 10 mM | 0.197 mL | 0.985 mL | 1.9699 mL |
Step 1: Enter information below (Recommended: An additional animal making an allowance for loss during the experiment)
 g
g
 μL
μL

Step 2: Enter the in vivo formulation (This is only the calculator, not formulation. Please contact us first if there is no in vivo formulation at the solubility Section.)
Calculation results:
Working concentration: mg/ml;
Method for preparing DMSO master liquid: mg drug pre-dissolved in μL DMSO ( Master liquid concentration mg/mL, Please contact us first if the concentration exceeds the DMSO solubility of the batch of drug. )
Method for preparing in vivo formulation: Take μL DMSO master liquid, next addμL PEG300, mix and clarify, next addμL Tween 80, mix and clarify, next add μL ddH2O, mix and clarify.
Method for preparing in vivo formulation: Take μL DMSO master liquid, next add μL Corn oil, mix and clarify.
Note: 1. Please make sure the liquid is clear before adding the next solvent.
2. Be sure to add the solvent(s) in order. You must ensure that the solution obtained, in the previous addition, is a clear solution before proceeding to add the next solvent. Physical methods such as vortex, ultrasound or hot water bath can be used to aid dissolving.
3. All of the above co-solvents are available for purchase on the GlpBio website.
Quality Control & SDS
- View current batch:
- Purity: >95.00%
- COA (Certificate Of Analysis)
- SDS (Safety Data Sheet)
- Datasheet
-
Related Biological Data

Cellular internalization and localization of MoS2 nanosheets. A) Representative images of MoS2 nanosheets and brightfield were taken by confocal microscopy; B) The fluorescence intensity of FITC-BSA labeled MoS2 nanosheets was quantified by flow cytometry.
Cells were exposed to FITC-BSA labeled MoS2 nanosheets and simultaneously treated with different cellular internalization inhibitors, including Cyto D (GLPBIO, Montclair, CA, USA), Nystatin, chlorpromazine and 4-PBA.
Small, 2023: 2208063. PMID: 36908089 IF: 15.1536 -
Related Biological Data

Hydrogels with strain-enhanced stress relaxation regulate myocardin related transcription factor (MRTF) nuclear localization through the polymerization of F-actin. H) Schematics illustrating the inhibitory effect of Cyto-D on actin polymerization.
Cytochalasin (Cyto)-D (Glpbio, GC13440)
Small 20.9 (2024): 2305218. PMID: 37847903 IF: 13.3003 -
Related Biological Data

The engineered strains enhanced the activation of the STING pathway in innate immune cells, blocked by cytochalasin D. (H and I) THP1-Dual cells were pretreated with cytochalasin D (10 μM) or media for 1 h; the ratio of CIBT4523 (H) or CIBT4712 (I) to THP1-Dual cells is shown as indicated.
Additionally, to perform the cytochalasin D (GC13440, GlpBio, US) assay, these compounds (10 μM) were pretreated with the indicated cells for 1 h before each strain was added.
Research 6 (2023): 0102. PMID: 37011280 IF: 11.0003 -
Related Biological Data

Transcriptome sequencing of macrophages on different fibers.(e & f & g) Effects of addition of cytochalasin D on ATP production (e) and TNF-α (f) and IL-10 (g) secretion of macrophages.
Cytochalasin D (GLPBIO, USA) with a concentration of 0.1 μg/mL was added to the inhibition group and cultured for 24 h.
Acta Biomaterialia (2024). PMID: 38579918 IF: 9.7004 -
Related Biological Data

Confocal micrographs of MAC-T cells pretreated with or without indicated inhibitors, followed by 2 h of incubation with 20 μg/mL DiO-labeled S. aureus EVs.
Endocytosis inhibitors cytochalasin D (cyto D), methyl-β-cyclodextrin (MβCD), and dynasore were purchased from Glpbio (United States) and used at a final concentration of 2.5 μM, 4 mM, and 40 μM, respectively.
Microbiol Res (2023): 127421. IF: 5.0698 -
Related Biological Data
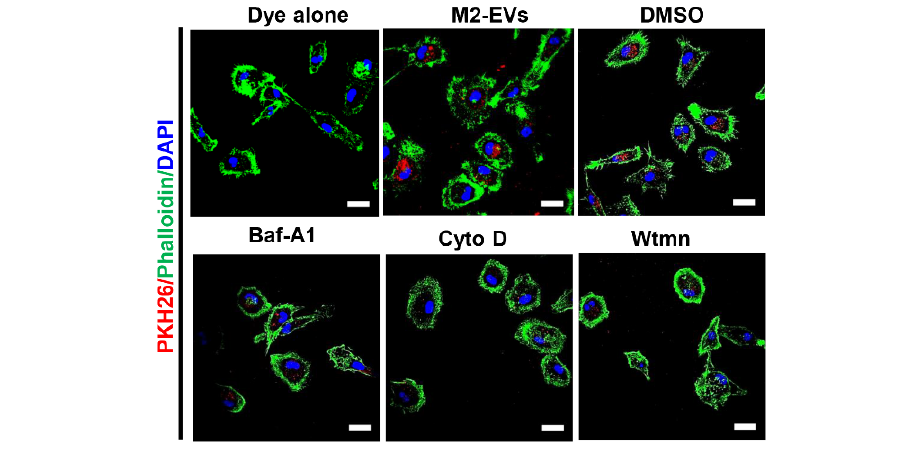
Isolation and evaluation of properties of M2-EVs in vitro.(E) Cells were pre-treated with DMSO, Baf-A1 (Bafilomycin A1), Cyto D (Cytochalasin D) or Wtmn (Wortmannin) for 15 min, and the incubated PKH26 labeled M2-EVs (Red) for 4 h.
To determine the cellular uptake mechanism of M2-EVs, cells were pretreated with inhibitors including Bafilomycin A1 (10 nM, Glpbio), Cytochalasin D (0.5 μM, Glpbio), and Wortmannin (0.5 μM, Glpbio) for 30 min, and then incubated with PKH26-169 labeled EVs.
bioRxiv (2022). PMID: 35792186
Average Rating: 5 (Based on Reviews and 22 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *



