Reactive O2/N2 Pathways
Products for Reactive O2/N2 Pathways
- Cat.No. Nombre del producto Información
-
GC41213
(±)10-HDHA
10hydroxy Docosahexaenoic Acid, (±)10HDoHE
(±)10-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.

-
GC41214
(±)11-HDHA
11hydroxy Docosahexaenoic Acid, (±)11-HDoHE
(±)11-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
GC40428
(±)11-HEDE
11-hydroxy-12,14-Eicosadienoic Acid
(±)11-HEDE is produced by non-enzymatic oxidation of 11,14-eicosadienoic acid.
-
GC40387
(±)11-HEPE
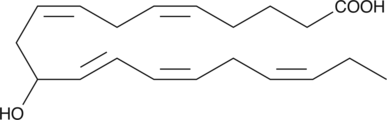
(±)11-HEPE is produced by non-enzymatic oxidation of eicosapentaenoic acid.
-
GC40467
(±)11-HETE
(±)11-Hydroxyeicosatetraenoic Acid
(±)11-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
GC40359
(±)12-HEPE
(±)12-HEPE is produced by non-enzymatic oxidation of EPA.

-
GC40429
(±)12-HETE
(±)-Hydroxyeicosatetraenoic Acid
(±) 12-HETE, un importante producto metabólico del ácido araquidónico mediante catálisis 12-LOX, inhibe la apoptosis celular de forma dependiente de la dosis.
-
GC48738
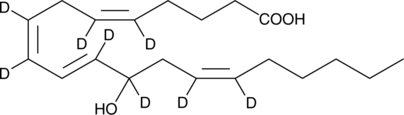
(±)12-HETE-d8
(±)12-Hydroxyeicosatetraenoic Acid-d8
-
GC41115
(±)12-HpETE
(±)12-HpETE is one of the six monohydroperoxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid and consists of an equal mixture of the R and S isomers.

-
GC41192
(±)13-HDHA
13hydroxy Docosahexaenoic Acid, (±)13-HDoHE
(±)13-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
GC40355
(±)13-HpODE
13-Hydroperoxylinoleic acid; Linoleic acid 13-hydroperoxide
(±)13-HpODE (ácido 13-hidroperoxilinoleico) es una mezcla racémica de hidroperóxidos, que se produce por la oxidación del ácido linoleico por la lipoxigenasa.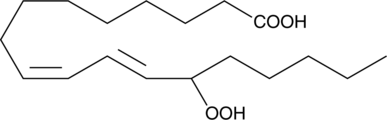
-
GC41193
(±)14-HDHA
14hydroxy Docosahexaenoic Acid, (±)14-HDoHE
(±)14-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
GC40420
(±)15-HEDE
(±)15-HEDE is produced by non-enzymatic oxidation of 11,14-eicosadienoic acid.

-
GC40361
(±)15-HEPE
(±)15-hydroxy Eicosapentaenoic Acid
(±)15-HEPE is produced by non-enzymatic oxidation of EPA.

-
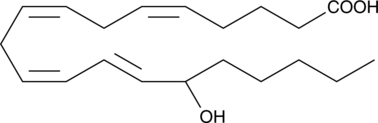
GC40433
(±)15-HETE MaxSpec® Standard
(±)15-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
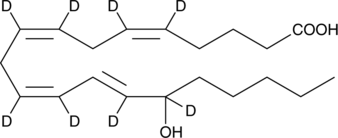
GC48739
(±)15-HETE-d8
(±)15-Hydroxyeicosatetraenoic Acid-d8
-
GC41196
(±)16-HDHA
16hydroxy Docosahexaenoic Acid, (±)16-HDoHE
(±)16-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
GC41197
(±)17-HDHA
17-hydroxy Docosahexaenoic Acid, 17-hydroxy DHA, (±)17-HDoHE
(±)17-HDHA is an autoxidation product of docosahexaenoic acid in vitro.
-
GC41198
(±)17-HDHA MaxSpec® Standard
17-hydroxy Docosahexaenoic Acid, 17-hydroxy DHA, (±)17-HDoHE
(±)17-HDHA is an autoxidation product of docosahexaenoic acid in vitro.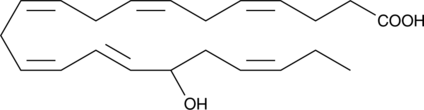
-
GC40362
(±)18-HEPE
(±)18-HEPE is produced by non-enzymatic oxidation of EPA.

-
GC41202
(±)4-HDHA
4hydroxy Docosahexaenoic Acid, (±)4HDoHE
(±)4-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.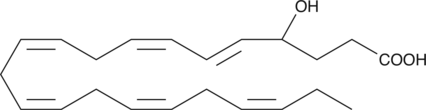
-
GC40364
(±)5-HEPE
(±)5-HEPE is produced by non-enzymatic oxidation of EPA.

-
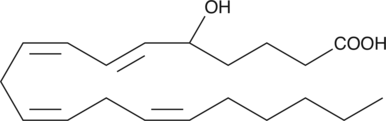
GC40439
(±)5-HETE
(±)5-Hydroxyeicosatetraenoic Acid
(±)5-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
GC40828
(±)5-HETE lactone
(±)5-Hydroxyeicosatetraenoic Acid lactone
(±)5-HETE lactone is a cyclic ester formed by acid-catalyzed nucleophilic addition of the C-5 hydroxyl to the C-1 carboxyl of (±)5-HETE.
-
GC40837
(±)5-HETE methyl ester
(±)-Hydroxyeicosatetraenoic Acid methyl ester
(±)5-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
GC40576
(±)5-iPF2α-VI
iPF2αI, Isoprostane F2α-I, 5-iso PGF2α-VI, 5-iso Prostaglandin F2α-VI
Isoprostanes are prostaglandin (PG)-like products of free-radical induced lipid peroxidation.
-
GC41204
(±)7-HDHA
7hydroxy Docosahexaenoic Acid, (±)7HDoHE
(±)7-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.

-
GC41205
(±)8-HDHA
8hydroxy Docosahexaenoic Acid, (±)8HDoHE
(±)8-HDHA is an autoxidation product of docosahexaenoic acid (DHA) in vitro.
-
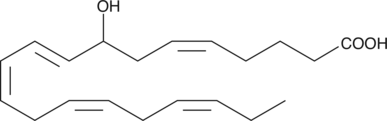
GC40366
(±)8-HEPE
(±)8-HEPE is produced by non-enzymatic oxidation of EPA.
-
GC40367
(±)9-HEPE
(±)9-HEPE is produced by non-enzymatic oxidation of EPA.

-
GC40443
(±)9-HETE
(±)9-Hydroxyeicosatetraenoic Acid
(±)9-HETE is one of the six monohydroxy fatty acids produced by the non-enzymatic oxidation of arachidonic acid.
-
GC46268
(±)9-HETE-d8
(±)9-Hydroxyeicosatetraenoic Acid-d8
A neuropeptide with diverse biological activities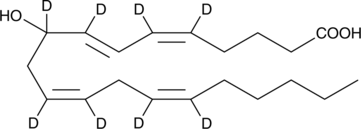
-
GC40356
(±)9-HpODE
(±)9-HpODE es un hidroperóxido lipídico de cadena larga, es un producto de la peroxidación del ácido linoleico.

-
GC19461
(±)13-HODE
(±)13-HODE is one of the two racemic monohydroxy fatty acids resulting from the non-enzymatic oxidation of linoleic acid.

-
GC42023
1-Palmitoyl-2-Arachidonoyl-sn-glycero-3-PC
1-Palmitoyl-2-Arachidonoyl-sn-glycero-3-Phosphocholine, PAPC, PC(16:0/20:4)
1-Palmitoyl-2-arachidonoyl-sn-glycero-3-PC (PAPC) is a phospholipid containing palmitic acid (16:0) and arachidonic acid (20:4) at the sn-1 and sn-2 positions, respectively, that is found in biological membranes.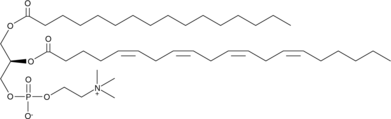
-
GC90811
1-Palmitoyl-d9-2-Arachidonoyl-sn-glycero-3-PC
Un estándar interno para la cuantificación de 1-palmitoil-2-arachidonoil-sn-glicero-3-PC.

-
GC42042
1-Stearoyl-2-15(S)-HpETE-sn-glycero-3-PE
1-Stearoyl-2-15(S)-HpETE-sn-glycero-3-phosphoethanolamine, 15(S)-HpETE-SAPE, 15(S)-hydroperoxyeicostetraenoic acid-SAPE
1-Stearoyl-2-15(S)-HpETE-sn-glycero-3-PE is a phospholipid that contains stearic acid at the sn-1 position and 15(S)-HpETE at the sn-2 position.
-
GC41868
10-Nitrooleate
10Nitrooleic Acid, 10nitro9transOctadecenoic Acid
El 10-nitrooleato (CXA-10), un Ácido graso nitro, tiene efectos potenciales en estados patolÓgicos en los que el estrés oxidativo, la inflamaciÓn, la fibrosis y/o la toxicidad tisular directa juegan un papel importante.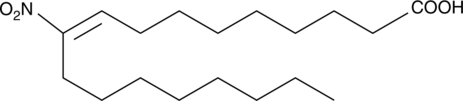
-
GC46403
10-Nitrooleate-d17
10Nitrooleic Acidd17, 10nitro9-trans-Octadecenoic Acidd17
A neuropeptide with diverse biological activities
-
GC40368
11(R)-HEPE
11(R)-HEPE is produced by the oxidation of EPA by 11(R)-LO.

-
GC40370
12(R)-HEPE
12(R)-HEPE is a monohydroxy fatty acid synthesized from EPA by the eggs of the sea urchin, S.

-
GC46420
13(S)-HODE-d4
An internal standard for the quantification of 13-HODE

-
GC40426
15(R)-HEDE
15(R)-Hydroxyeicosatetraenoic Acid
15(R)-HEDE is isolated by the chromatographic resolution of (±)15-HEDE.
-
GC46441
15(S)-HEPE-d5
15S-Hydroxyeicosapentaenoic Acid-d5
A neuropeptide with diverse biological activities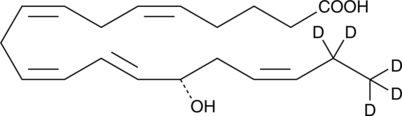
-
GC46442
15(S)-HETE-d8
15(S)-Hydroxyeicosatetraenoic Acid-d8
An internal standard for the quantification of 15-HETE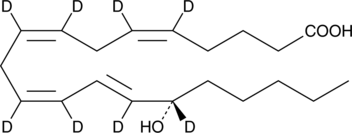
-
GC41928
15-deoxy-δ12,14-Prostaglandin J2 Glutathione
15deoxyΔ12,14PGJ2 Glutathione
15-deoxy-δ12,14-Prostaglandin J2 Glutathione (15-deoxy-δ12,14-PGJ2 Glutathione) is a non-enzymatic adduct formed from 15-deoxy-δ12,14-PGJ2 and glutathione.
-
GC40453
16(R)-HETE
16(R)-Hydroxyeicosatetraenoic Acid
Electrolyte and fluid transport in the kidney are regulated in part by arachidonic acid and its metabolites.

-
GC41207
17(R)-HDHA
17(R)hydroxy Docosahexaenoic Acid, 17(R)HDoHE
Resolvins are a group of polyhydroxylated metabolites of docosahexaenoic acid (DHA) found in the inflammatory exudates of aspirin-treated experimental animals.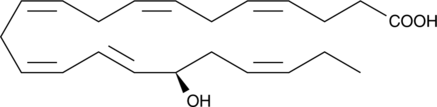
-
GC90081
2,3-dinor-8-iso Prostaglandin F1α
Un metabolito activo del ácido araquidónico y 8-iso PGF2α.

-
GC41210
22-HDHA
22-hydroxy Docosahexaenoic Acid, 22-OH DHA
22-HDHA is an oxidation product of docosahexaenoic acid.
-
GC42400
4-hydroperoxy 2-Nonenal
4HpNE
4-hydroxy Nonenal is a lipid peroxidation product derived from oxidized ω-6 polyunsaturated fatty acids, such as linoleic acid and arachidonic acid, that is widely used as a marker of oxidative stress.
-
GC40778
4-hydroxy Hexenal
4-HHE
4-hydroxy Hexenal is a lipid peroxidation product derived from oxidized ω-3 fatty acids such as DHA.

-
GC46656
4-hydroxy Hexenal-d3
4-HHE-d3
An internal standard for the quantification of 4-hydroxy hexenal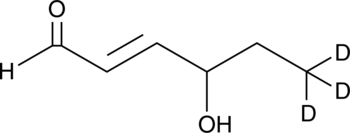
-
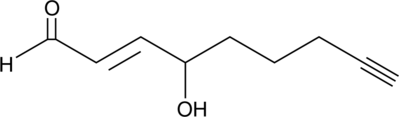
GC42411
4-hydroxy Nonenal Alkyne
Click Tag 4HNE Alkyne
4-hydroxy Nonenal (4-HNE) is a major aldehyde produced during the lipid peroxidation of ω-6 polyunsaturated fatty acids, such as arachidonic acid and linoleic acid.
-
GC42412
4-hydroxy Nonenal Glutathione (trifluoroacetate salt)
4HNEGSH
4-hydroxy Nonenal Glutathione (HNE-GSH) is a major adduct formed by the reaction of 4-HNE with GSH.
-
GC42413
4-hydroxy Nonenal Mercapturic Acid
Peroxidation of common ω-6 polyunsaturated fatty acids (PUFAs) such as linoleic acid, DGLA, and arachidonic acid can give rise to 4-HNE.

-
GC46660
4-hydroxy Nonenal Mercapturic Acid-d3
A neuropeptide with diverse biological activities
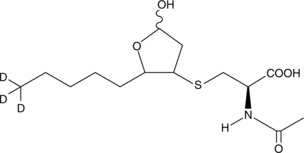
-
GC42464
4-oxo-2-Nonenal
4-ONE
4-hydroxy Nonenal is a lipid peroxidation product derived from oxidized ω-6 polyunsaturated fatty acids such as arachidonic acid and linoleic acid.

-
GC46674
4-oxo-2-Nonenal-d3
4ONEd3
An internal standard for the quantification of 4oxo-2nonenal
-
GC40053
5α,6α-epoxy Cholestanol
NSC 18176
An oxysterol and a metabolite of cholesterol produced by oxidation
-
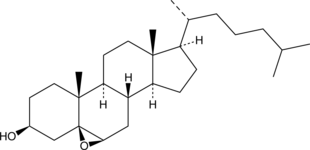
GC40418
5β,6β-epoxy Cholestanol
NSC 148940
5β,6β-epoxy Cholestanol is an oxidative metabolite of cholesterol that is formed via radical and non-radical oxidation of cholesterol at the 5,6-double bond.
-
GC40459
5(R)-HETE
5(R)-Hydroxyeicosatetraenoic Acid
5(R)-HETE is a rare lipoxygenase product of arachidonic acid.
-
GC46679
5(S)-HETE-d8
5(S)-Hydroxyeicosatetraenoic Acid-d8
An internal standard for the quantification of 5-HETE
-
GC40201
7β-hydroxy Cholesterol-d7
7α-hydroxycholesterol-d7
7β-hydroxy Cholesterol-d7 is intended for use as an internal standard for the quantification of 7β-hydroxy cholesterol by GC- or LC-MS.
-
GC40572
7-keto Cholesterol
SC-4722
El colesterol 7-ceto, oxisterol tóxico, inhibe el paso limitante de la biosíntesis de ácidos biliares colesterol 7 alfa-hidroxilasa, así como también inhibe fuertemente la HMG-CoA reductasa (la enzima limitante de la biosíntesis del colesterol).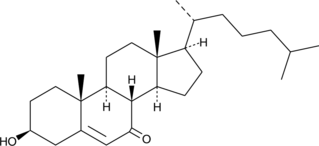
-
GC40587
8,12-iso-iPF2α-VI
8,12-iso-Isoprostane-F2α-VI, 12-iso-5,6E,14Z-PGF2α, 12iso5,6E,14ZProstaglandin F2α
8,12-iso-iPF2α-VI is an isoprostane produced by non-enzymatic, free radical-induced peroxidative damage to membrane lipids.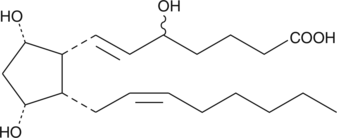
-
GC41137
8,12-iso-iPF2α-VI-1,5-lactone
F2 isoprostanes (F2-iPs) are thought to arise from the free radical-mediated peroxidation of phospholipid-bound arachidonic acid.

-
GC46743
8,12-iso-iPF2α-VI-d11
8,12-iso-Isoprostane-F2α-VI-d11, 12-iso-5,6E,14Z-PGF2α-d11, 12-iso-5,6E,14Z-Prostaglandin F2α-d11
A neuropeptide with diverse biological activities
-
GC41247
8-iso Misoprostol
Misoprostol is a widely sold analog of prostaglandin E1 (PGE1) which has potent but relatively non-selective agonist activity with respect to the prostanoid EP receptor subgroup.

-
GC41138
8-iso Prostaglandin A1
8-epi PGA1, 8-iso PGA1
8-iso Prostaglandin A1 (8-iso PGA1) is an isoprostane and a member in a large family of prostanoids of non-cyclooxygenase origin.
-
GC18781
8-iso Prostaglandin A2
A2 Isoprostane, 15-A2t-IsoP, 15-A2t-Isoprostane, 8-epi PGA2, 8-iso PGA2
Isoprostanes are prostaglandin (PG)-like compounds produced in vivo by free radical-catalyzed peroxidation of arachidonoyl-containing lipids.
-
GC42628
8-iso Prostaglandin A2-biotin
8iso PGA2biotin
Isoprostanes are prostaglandin (PG)-like compounds produced in vivo by free radical-catalyzed peroxidation of arachidonoyl-containing lipids.
-
GC18737
8-iso Prostaglandin E1
8epi PGE1, 8iso PGE1, Ovinonic acid
Isoprostanes are a family of prostanoid molecules of non-cyclooxygenase origin.
-
GC41425
8-iso Prostaglandin E2
8iso PGE2, 8epi PGE2
La 8-iso prostaglandina E2 (iPE2-III) es un miembro de la clase de prostanoides isoprostano.
-
GC42629
8-iso Prostaglandin E2 isopropyl ester
8iso PGE2 isopropyl ester
8-iso PGE2 isopropyl ester is a more lipophilic form of the free acid, 8-iso PGE2.
-
GC46745
8-iso Prostaglandin E2-d4
8epi PGE2d4, 8iso PGE2d4
A neuropeptide with diverse biological activities
-
GC41437
8-iso Prostaglandin F1α
8epi PGF1α
8-iso PGF1α is an isoprostane that was first identified in human semen.
-
GC46746
8-iso Prostaglandin F1α-d9
8epi PGF1αd9
A neuropeptide with diverse biological activities
-
GC41438
8-iso Prostaglandin F1β
8epi9βPGF1α, 8iso PGF1β
8-iso PGF1β is a potential autoxidation product of DGLA.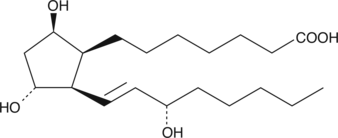
-
GC41405
8-iso Prostaglandin F2α Ethanolamide
iPF2αIII Ethanolamide, 8Isoprostane Ethanolamide, 8iso PGF2α Ethanolamide, 8epi PGF2α Ethanolamide
It has been reported that anandamide (AEA) can be used directly by cyclooxygenase-2 and specific prostaglandin H2 (PGH2) isomerases to produce ethanolamide congeners of the classical PGs, including PGF2α.
-
GC46747
8-iso Prostaglandin F2α-d4
iPF2αIIId4, 8Isoprostaned4, 15F2tIsoprostaned4, 8epi PGF2αd4
An internal standard for the quantification of 8iso prostaglandin F2α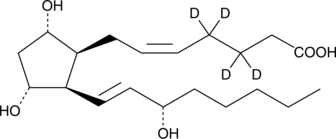
-
GC90100
8-iso Prostaglandin F2β
Un isómero de PGF2α producido por la peroxidación radical libre del ácido araquidónico.

-
GC18829
8-iso Prostaglandin F3α
8-epi PGF3α
8-iso PGF3α is an isoprostane produced from the free-radical peroxidation of EPA.

-
GC40588
8-iso-13,14-dihydro-15-keto Prostaglandin F2α
8iso13,14dihydro15keto PGF2α
8-iso-13,14-dihydro-15-keto Prostaglandin F2α (8-iso-13,14-dihydro-15-keto PGF2α) is a metabolite of the isoprostane, 8-isoprostane (8-iso PGF2α), in rabbits, monkeys and humans.
-
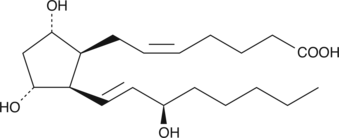
GC40589
8-iso-15(R)-Prostaglandin F2α
8iso15epi PGF2α
8-iso-15(R) PGF2α is one member of a large family of prostaglandin-like eicosanoids formed by the free radical peroxidation of arachidonic acid in membrane phospholipids.
-
GC40608
8-iso-15-keto Prostaglandin E2
8epi15keto PGE2, 8iso15keto PGE2
8-iso-15-keto Prostaglandin E2 (8-iso-15-keto PGE2) is an isoprostane, one member of a large family of biomarkers produced by the free radical peroxidative degradation of membrane lipids.
-
GC41426
8-iso-15-keto Prostaglandin F2α
8epi15keto PGF2α, 8iso15keto PGF2α
8-iso-15-keto Prostaglandin F2α (8-iso-15-keto PGF2α) is a metabolite of the isoprostane 8-iso PGF2α in rabbits, monkeys, and humans.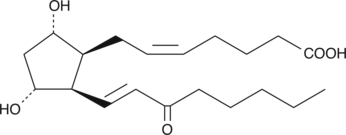
-
GC41427
8-iso-15-keto Prostaglandin F2β
8epi15keto PGF2α, 8iso15keto PGF2α
8-iso Prostaglandin F2β (8-iso PGF2β) is an isomer of PGF2α of non-enzymatic origin.
-
GC40332
8-iso-16-cyclohexyl-tetranor Prostaglandin E2
8-iso-16-cyclohexyl-tetranor PGE2
8-iso Prostaglandin E2 (8-iso PGE2) is one of several isoprostanes produced from polyunsaturated fatty acids during lipid peroxidation.
-
GC40991
8-iso-17-phenyl trinor Prostaglandin F2α
8-iso-17-phenyl PGF2β
8-iso-17-phenyl trinor Prostaglandin F2α (8-iso-17-phenyl trinor PGF2α) is the C-8 epimer of bimatoprost (free acid), a metabolically stable analog of PGF2α.
-
GC40992
8-iso-17-phenyl trinor Prostaglandin F2β
8-iso-17-phenyl PGF2β
Bimatoprost (free acid) is a metabolically stable analog of prostaglandin F2α (PGF2α) and a potent agonist for the FP receptor.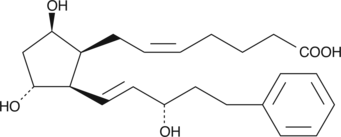
-
GC92043
9(10)-Nitrooleate
9(10)-Nitrooleic Acid; OA-NO2; 9(10)-nitro-9-trans-Octadecenoic Acid
9(10)-Nitrooleate es una mezcla de las moléculas de señalización lipíendde nitroalqueno 9-nitrooleato.
-
GC46748
9(E),11(E)-12-nitro Conjugated Linoleic Acid
9(E),11(E)-12-nitro CLA, 12-NO2-CLA
A nitrated fatty acid
-
GC46749
9(E),11(E)-9-nitro Conjugated Linoleic Acid
9E,11E-9-nitro CLA
A nitrated fatty acid
-
GC46751
9(R)-HETE-d8
9(R)-Hydroxyeicosatetraenoic Acid-d8
A neuropeptide with diverse biological activities
-
GC46755
9(S)-HODE-13C18
(+)-α-Dimorphecolic Acid
A neuropeptide with diverse biological activities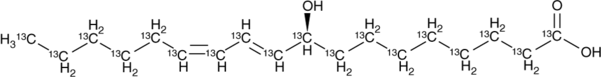
-
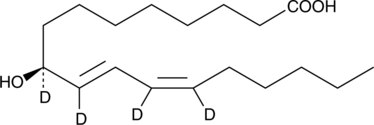
GC40250
9(S)-HODE-d4 MaxSpec® Standard
9(S)-HODE-d4 is intended for use as an internal standard for the quantification of 9(S)-HODE by GC- or LC-mass spectrometry.
-
GC42649
9-Nitrooleate
9Nitrooleic Acid, 9nitro9transOctadecenoic Acid
Nitrated unsaturated fatty acids, such as 10- and 12-nitrolinoleate, cholesteryl nitrolinoleate, and nitrohydroxylinoleate, represent a new class of endogenous lipid-derived signalling molecules.
-
GC46760
9-Nitrooleate-d17
9Nitrooleic Acidd17, 9nitro9-trans-Octadecenoic Acidd17
A neuropeptide with diverse biological activities
-
GC46761
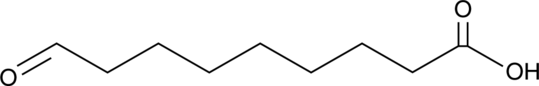
9-Oxononanoic Acid
Azelaaldehydic Acid
An oxidized fatty acid