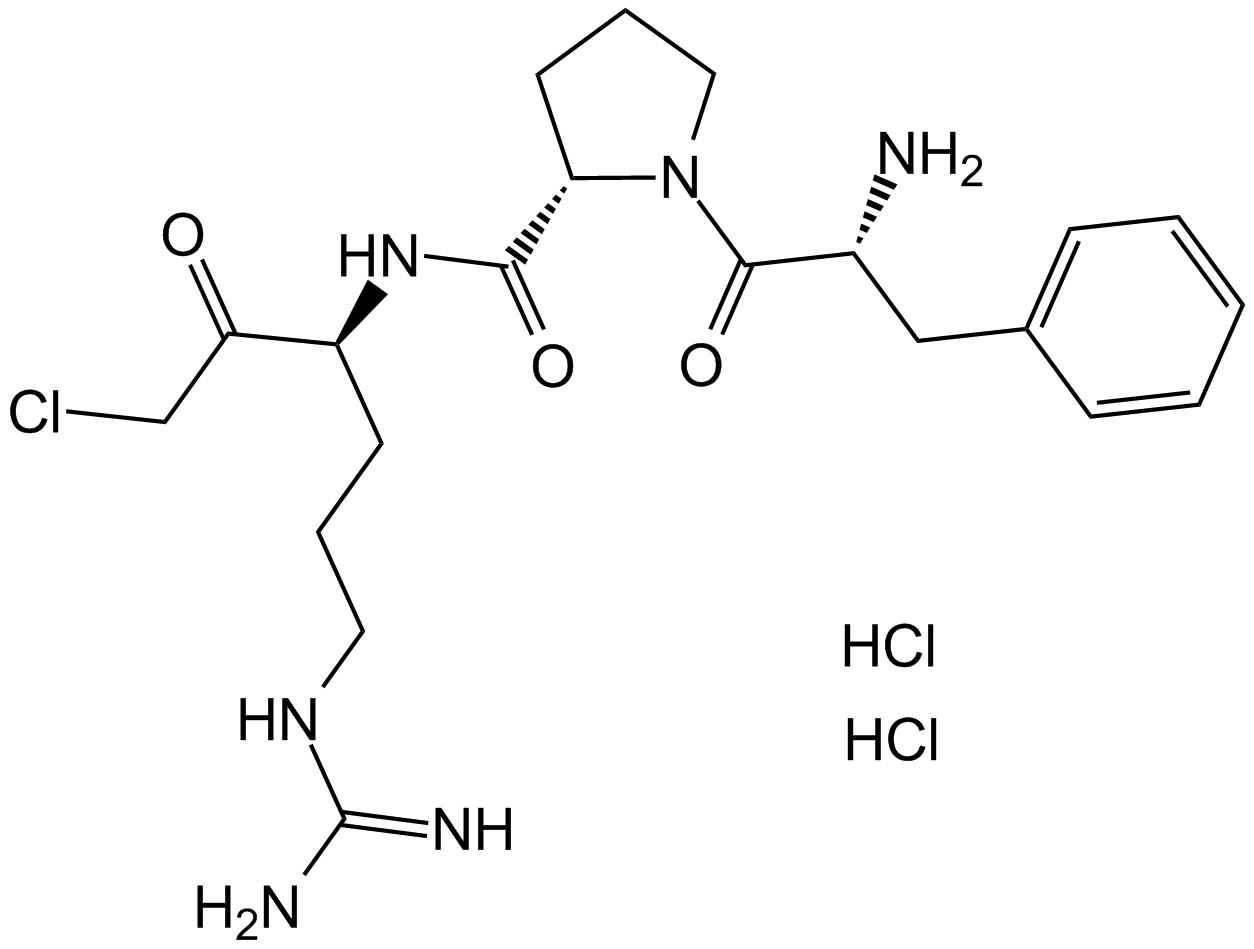
PPACK Dihydrochloride (Synonyms: PPACK Dihydrochloride) |
| Catalog No.GC10277 |
Inhibidor de la trombina.
Products are for research use only. Not for human use. We do not sell to patients.
Sample solution is provided at 25 µL, 10mM.
PPACK is a potent selective and irreversible thrombin inhibitor that inhibits human alpha thrombin with a Ki value of 0.24 nM. PPACK inhibit the binding of rt-PA to plasma protease inhibitors in vitro[1-3]. It forms a covalent bond to the thrombin active site Ser and cross-links with His57 at the active site to form a tetrahedral PPack-thrombin complex. PPACK completely inhibited changes in fibrin degradation products, plasminogen and alpha 2-antiplasmin[4-6].
Coincubation with PPACK(0.2 mM;2h) reduced the increase in endothelial permeability induced by plasmin by 59% in bovine aortic endothelial cells[7]. Heme oxygenase (HO)-1 is a stress-inducible rate-limiting enzyme in heme degradation that confers cytoprotection against oxidative injury. Pretreatment of cells with PPACK(24h; 30 nM) effectively antagonized the potentiating effect of thrombin on HO-1 expression[8].
PPACK(bolus dose of 10-100 μg/kg or infusion of 0.25-1.0 μg/kg/min) pretreatment can inhibit thrombine-induced cerebral thromboembolism in rabbits[9].
References:
[1]. Mohler MA, Refino CJ, et,al. D-Phe-Pro-Arg-chloromethylketone: its potential use in inhibiting the formation of in vitro artifacts in blood collected during tissue-type plasminogen activator thrombolytic therapy. Thromb Haemost. 1986 Oct 21;56(2):160-4. PMID: 2433785.
[2]. Bode W, Mayr I, et,al. The refined 1.9 A crystal structure of human alpha-thrombin: interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Pro-Trp insertion segment. EMBO J. 1989 Nov;8(11):3467-75. doi: 10.1002/j.1460-2075.1989.tb08511.x. PMID: 2583108; PMCID: PMC401503.
[3]. Lyon ME, Fine JS, et,al. D-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK): alternative anticoagulant to heparin salts for blood gas and electrolyte specimens. Clin Chem. 1995 Jul;41(7):1038-41. PMID: 7600685.
[4]. Kovach IM, Kelley P, et,al. Proton bridging in the interactions of thrombin with small inhibitors. Biochemistry. 2009 Aug 4;48(30):7296-304. doi: 10.1021/bi900098s. PMID: 19530705; PMCID: PMC2800789.
[5]. Kettner C, Shaw E. D-Phe-Pro-ArgCH2C1-A selective affinity label for thrombin. Thromb Res. 1979;14(6):969-73. doi: 10.1016/0049-3848(79)90014-8. PMID: 473131.
[6]. Nienaber VL, Mersinger LJ, et,al. Structure-based understanding of ligand affinity using human thrombin as a model system. Biochemistry. 1996 Jul 30;35(30):9690-9. doi: 10.1021/bi952164b. PMID: 8703940.
[7]. Rabbani LE, Johnstone MT, et,al. PPACK attenuates plasmin-induced changes in endothelial integrity. Thromb Res. 1993 Jun 15;70(6):425-36. doi: 10.1016/0049-3848(93)90085-3. PMID: 8362368.
[8].Liu JF, Hou SM, et,al. Thrombin induces heme oxygenase-1 expression in human synovial fibroblasts through protease-activated receptor signaling pathways. Arthritis Res Ther. 2012 Apr 27;14(2):R91. doi: 10.1186/ar3815. PMID: 22541814; PMCID: PMC3446465.
[9]. Liu JT, Paul W, et,al. Thrombin inhibitors and anti-coagulants on thrombin-induced embolisation in rabbit cranial vasculature. Eur J Pharmacol. 1994 Oct 24;264(2):183-90. doi: 10.1016/0014-2999(94)00464-1. PMID: 7851481.
Average Rating: 5 (Based on Reviews and 30 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















