Talampanel (Synonyms: GYKI 53773, LY 300164) |
| Catalog No.GC19346 |
Talampanel (LY300164) es un antagonis oral y selectivo del receptor de α-amino-3-hidroxi-5-metil-4-isoxazolpropionato (AMPA) con actividad anticonvulsiva.
Products are for research use only. Not for human use. We do not sell to patients.
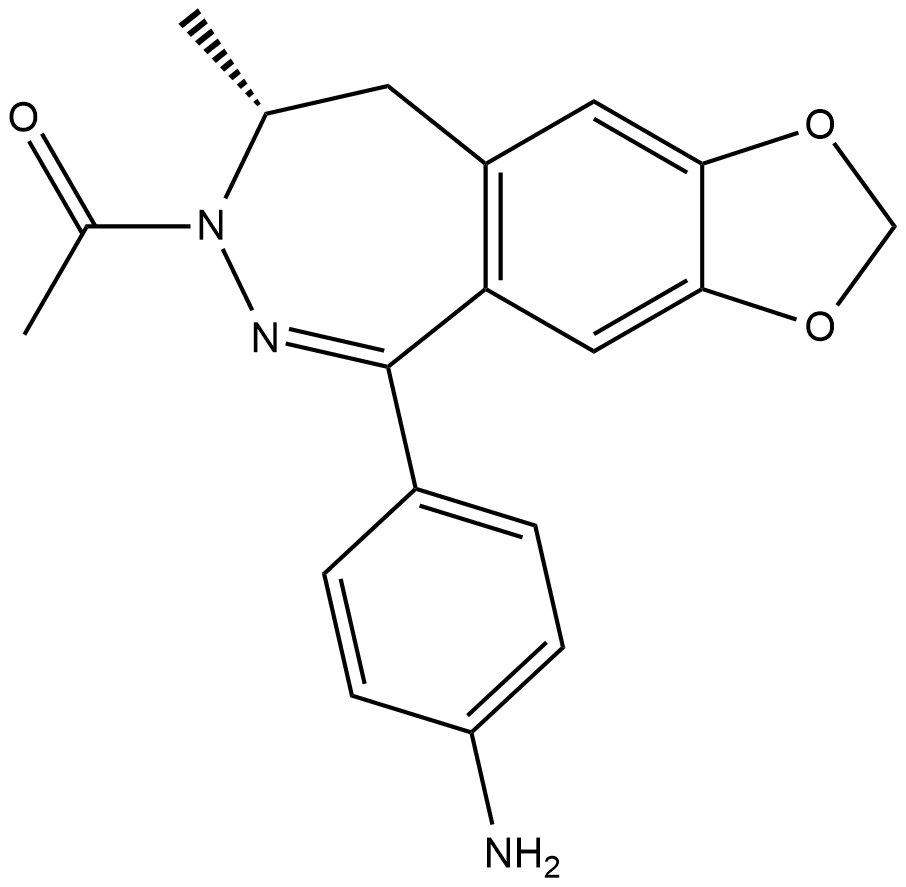
Cas No.: 161832-65-1
Sample solution is provided at 25 µL, 10mM.
Talampanel is a potent and selective AMPA-receptor antagonist, is a potential new antiepileptic drug (AED). Target: AMPA [1]in vitro: Talampanel is a glutamate receptor inhibitor with anti-seizure activity.in vivo: Talampanel reduces motoneuronal calcium in a mouse model of ALS, but its effi cacy declines as the disease progresses, suggesting that medication initiation in the earlier stages of the disease might be more effective. [2]
References:
[1]. Langan YM, et al. Talampanel, a new antiepileptic drug: single- and multiple-dose pharmacokinetics and initial 1-week experience in patients with chronic intractable epilepsy. Epilepsia. 2003 Jan;44(1):46-53.
[2]. Paizs M, et al. Talampanel reduces the level of motoneuronal calcium in transgenic mutant SOD1 mice only if applied presymptomatically. Amyotroph Lateral Scler. 2011 Sep;12(5):340-344.
Average Rating: 5 (Based on Reviews and 27 reference(s) in Google Scholar.)
GLPBIO products are for RESEARCH USE ONLY. Please make sure your review or question is research based.
Required fields are marked with *




















